45674
Erythromycin
tested according to Ph. Eur.
Synonym(s):
Erythromycinum
About This Item
Recommended Products
Agency
EPA 1694
USP/NF
tested according to Ph. Eur.
Quality Level
form
solid
color
white to faint yellow
solubility
H2O: soluble 2 mg/mL
acetone: freely soluble
acetonitrile: freely soluble
alcohol: soluble
amyl acetate: moderately soluble
chloroform: soluble
diethyl ether: soluble
ethyl acetate: freely soluble
antibiotic activity spectrum
Gram-negative bacteria
Gram-positive bacteria
application(s)
environmental
Mode of action
protein synthesis | interferes
SMILES string
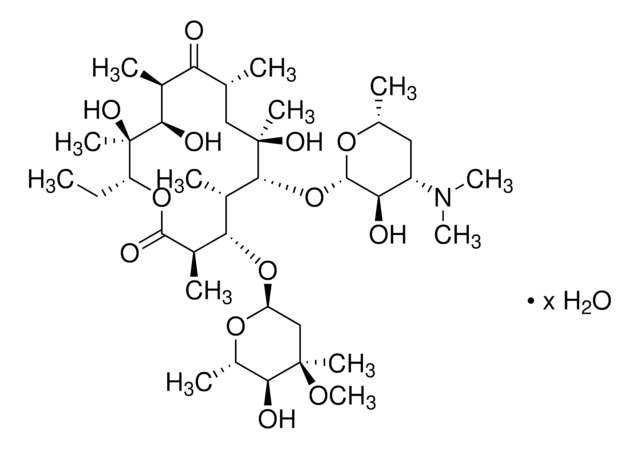
CC[C@H]1OC(=O)[C@H](C)[C@@H](O[C@H]2C[C@@](C)(OC)[C@@H](O)[C@H](C)O2)[C@H](C)[C@@H](O[C@@H]3O[C@H](C)C[C@@H]([C@H]3O)N(C)C)[C@](C)(O)C[C@@H](C)C(=O)[C@H](C)[C@@H](O)[C@]1(C)O
InChI
1S/C37H67NO13/c1-14-25-37(10,45)30(41)20(4)27(39)18(2)16-35(8,44)32(51-34-28(40)24(38(11)12)15-19(3)47-34)21(5)29(22(6)33(43)49-25)50-26-17-36(9,46-13)31(42)23(7)48-26/h18-26,28-32,34,40-42,44-45H,14-17H2,1-13H3/t18-,19-,20+,21+,22-,23+,24+,25-,26+,28-,29+,30-,31+,32-,34+,35-,36-,37-/m1/s1
InChI key
ULGZDMOVFRHVEP-RWJQBGPGSA-N
Gene Information
human ... ABCB1(5243) , CYP3A4(1576) , MLNR(2862)
mouse ... Abcb1a(18671) , Abcb1b(18669)
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
Biochem/physiol Actions
Antimicrobial Spectrum: This product acts against both gram-negative and gram-positive bacteria.
Packaging
Caution
Preparation Note
Other Notes
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Protein synthesis is a complex, multi-step process involving many enzymes as well as conformational alignment. However, the majority of antibiotics that block bacterial protein synthesis interfere with the processes at the 30S subunit or 50S subunit of the 70S bacterial ribosome.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service