Pyrogen Testing
Testing for pyrogens is a critical step in ensuring parenteral pharmaceutical product and medical device safety. It is part of the mandatory release tests to avoid life-threatening fever reactions induced by pyrogenic substances. The monocyte activation test (MAT) can detect both endotoxin and non-endotoxin pyrogens in one in vitro test.
Read more about
Monocyte Activation Test (MAT)
Used to detect both endotoxins and non-endotoxin pyrogens in parenteral products, such as pharmaceuticals and medical devices, the MAT gives an in vitro alternative to conventional animal testing in accordance with regulatory guidelines.
The Rabbit Pyrogen Test and the Limulus Amebocyte Lysate (LAL) test are broadly used for pyrogen detection. Both methods use animals and show some limitations. The rabbit pyrogen test shows a lack of robustness as an animal reaction can differ greatly from a human reaction. In the LAL test, only endotoxins are detected causing a safety risk by ignoring non-endotoxin pyrogens that could be present in the tested sample.
To overcome these limitations, the Monocyte-Activation Test (MAT) was introduced in the European Pharmacopoeia in 2010 as a compendial method to replace the Rabbit Pyrogen Test (EP Chapter 2.6.30) and mentioned in FDA guidance for industry.
Please Note: The European Pharmacopoeia Commission took the decision to put an end to the rabbit pyrogen test in accordance with the 3Rs principle considering that the MAT is the best alternative option.
Read the article and discuss further with our Experts to start the move.

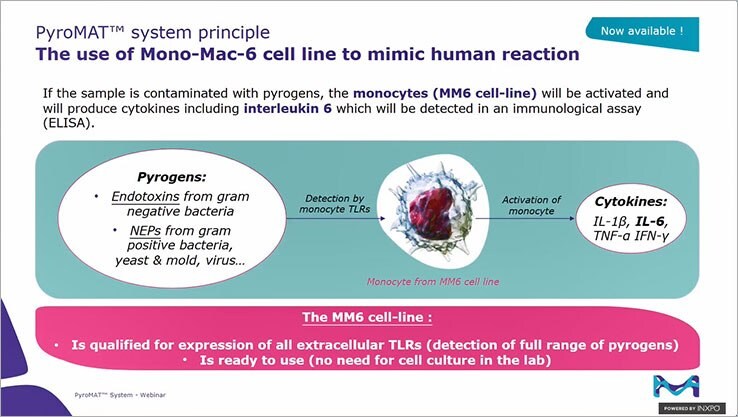
PyroMAT® in vitro test to detect endotoxin and non-endotoxin pyrogens
The PyroMAT® system is based on the Mono-Mac-6 cell line and IL-6 read-out. It offers all the advantages of the monocyte activation test combined with the benefits of using a cell line.
- Detection of a broad range of pyrogens: patient safety is ensured if the full range of pyrogens are tested. Like the rabbit pyrogen test (RPT), MAT is effective for both endotoxin and non-endotoxin pyrogen detection.
- Extension of the range of products that can be tested: the most frequently applied methods, RPT, Bacterial Endotoxin Test (BET) or LAL, are limited in the product types they are able to test. The MAT offers more flexibility regarding its applications.
- In vitro assay that mimics the human immune reaction: for a robust predictive model that reduces animal consumption.
- Compliance with international regulations and guidelines: in line with ethical trends of industry and regulatory authorities to decrease the use of animal-based testing.
- Standardized reactivity and high sensitivity (LOD 0.05 EU/mL). Convenience of a ready-to-use cell line reduces laborious lab work and avoids the need for a cell culture laboratory.
- Qualified cells: in addition to being cited in the international validation of MAT, Mono-Mac-6 cells are qualified for the expression of all surface Toll-Like Receptors (TLRs) to ensure the detection of a broad range of pyrogens.
Read more about Non-endotoxin Pyrogen Positive Controls.
Non-endotoxin Pyrogen Positive Controls
The European Pharmacopeia Chapter 2.6.30 (Monocyte Activation Test) requires that the preparatory testing includes "at least 2 non-endotoxin ligands for toll-like receptors […]. The choice of non-endotoxin pyrogens used should reflect the most likely contaminants(s) of the preparation being examined."
To respond to this requirement, we are providing an extended range of positive controls:
- Reflecting several types of contaminants usually found in pharmaceutical production processes (Gram-negative and Gram-positive bacteria, viruses and mycoplasma).
- Targeting several monocytic toll like receptors (TLR)

- Detection of a wide spectrum of pyrogens: like the rabbit pyrogen test (RPT), the MAT detects both endotoxins and NEPs.
- Extended range of products that can be tested: the most frequently applied methods, RPT, Bacterial Endotoxin Test (BET) and LAL, are limited in the product types they can be used with. The MAT offers greater flexibility regarding its applications.
- An in vitro assay that mimics the human immune reaction: for a robust predictive model that reduces animal consumption.
- Compliance with international regulations and guidelines: in line with ethical trends of industry and regulatory authorities to decrease the use of animal-based testing.
- Cryopreserved blood pooled from 8 donors: to be as close as possible to the human immune reaction to pyrogens.
Optimize or simplify your pyrogen testing method for an easy validation and cost-effective testing with our service offerings:
- Application services
- Validation services
- Training services
Comparison of rabbit, LAL and MAT pyrogen tests |
|---|
Lacking resources for MAT implementation and validation. We'll do it for you!
Feasibility study, method development, validation & training services to support your pyrogen testing implementation, discuss with our Pyrogen Testing Experts.
Related Product Resources
- Brochure: PyroMAT® and PyroDetect
Used to detect a broad range of pyrogens in parenteral products such as pharmaceuticals, biopharmaceuticals or medical devices, the MAT gives an in vitro alternative to conventional animal testing in accordance with regulatory guidelines.
- Article: Pyrogen Contamination Risk by Dr. Tim Sandle
Unlike the LAL test, the MAT is a powerful in vitro test for the detection of both endotoxin and non-endotoxin pyrogens (NEPs) acting via the toll-like receptors (TLRs) pathway.
- Data Sheet: Validation Method - PyroMAT® System
The Monocyte Activation Test (MAT) was introduced in the European Pharmacopeia (Chapter 2.6.30) in 2010, as a compendial method that can be used to replace the Rabbit Pyrogen Test (RPT).
- Infographic: MAT Implementation Workflow
Implementation of our Monocyte Activation Test (MAT), the PyroMAT® system.
- Poster: Pyrogen Detection in Pharma QC: Moving Away from the RPT
We have demonstrated that the MM6 cells are suitable for the detection of various NEPs targeting different monocytic toll-like receptors, making MM6 cell line-based MAT suitable for RPT replacement.
- Application Note: Detection of NEP by MAT Using the PyroMAT® System
The monocyte activation test (MAT) is the human in vitro alternative to the rabbit pyrogen test, and allows the detection of the full range of pyrogens, including endotoxins and non-endotoxin pyrogens (NEPs).
- Application Note: Detection of Pyrogens in Vaccines with PyroMAT® System
The PyroMAT™ System uses cryo-preserved Mono-Mac-6 (MM6) human monocytic cells as a source of monocytes. The response to pyrogenic substances is determined by measurement of Interleukin-6 (IL-6) produced by the MonoMac 6 cells.
- White Paper: MAT Statistical Analysis
The use of different sources of monocytes (blood vs cell line) leads to a different reactivity profile that requires the use of different regression models for each of these solutions.
- Questions & Answers about MAT. Dr. Tim Sandle Reponds
Risk considerations for the presence of pyrogens and the need for the MAT test in pharmaceutical processing.
- White Paper: Monocyte Activation Test
In this context, the in vitro pyrogen test based on human cells offers a valuable alternative to the rabbit pyrogen test.
- Application Note: Detection of Pyrogens in Hormone-based Drugs
For the quantification of the pyrogenic load of a sample, method A in accordance with the European Pharmacopeia can be conducted.
- Application Note: Detection of Pyrogens in FBS with PyroMAT® System
The response to pyrogenic substances is determined by measurement of Interleukin-6 (IL-6) produced by the Mono-Mac-6 cells. For this purpose, the ELISA-microplate supplied in the kit is coated with an antibody specific to IL-6.
To continue reading please sign in or create an account.
Don't Have An Account?