G7126
Glycine
ReagentPlus®, ≥99% (HPLC)
Synonym(s):
Aminoacetic acid, Aminoethanoic acid, Glycocoll
About This Item
Recommended Products
Quality Level
product line
ReagentPlus®
Assay
≥99% (HPLC)
form
powder
technique(s)
western blot: suitable
impurities
≤0.01% Ammonia
loss
≤0.2% loss on drying
color
white to off-white
pKa (25 °C)
(1) 2.35, (2) 9.60
2.35
mp
240 °C (dec.) (lit.)
solubility
H2O: 200 mg/mL, clear, colorless to faintly yellow
cation traces
≤0.01% (Ammonia (NH3))
functional group
amine
carboxyl
storage temp.
room temp
SMILES string
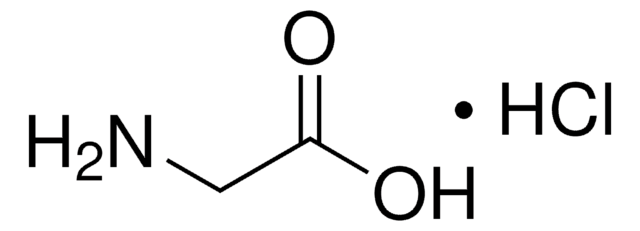
NCC(O)=O
InChI
1S/C2H5NO2/c3-1-2(4)5/h1,3H2,(H,4,5)
InChI key
DHMQDGOQFOQNFH-UHFFFAOYSA-N
Gene Information
rat ... Grin2a(24409)
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Beyond its vital biological functions, glycine demonstrates remarkable versatility in various research applications. Its application as a biochemical reagent makes it invaluable in numerous assays and procedures. Its buffering capacity allows it to play a critical role in protein analysis techniques like SDS-PAGE and Western Blotting, chromatography, and cell culture. Glycine′s zwitterionic nature makes it an effective buffer across a range of pH values. In immunology research, it is widely used in the preparation of buffers for Western Blotting and other techniques. Additionally, its compatibility with various enzymes makes it useful in enzymatic assays like lactate determination. Furthermore, glycine contributes to the formulation of buffers for protein stabilization, pH control, and enzymatic reactions.
Application
- Glycine has been added in the transfer buffer during western blotting procedure.
- It has been used in the solution prepared for dissolving formazan crystals in the MTT (3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyltetrazolium bromide) cytotoxicity assay.
- It has been used for terminating the rat basophilic leukemia (RBL) assay.
Biochem/physiol Actions
Features and Benefits
- Suitable for Cell Biology and Biochemical research
- High-quality compound suitable for multiple research applications
Other Notes
Legal Information
comparable product
related product
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Neoplastic cells are highly dependent on the de novo synthesis of nucleotides to maintain sufficient pools to support DNA replication and the production of RNA.
Protocols
Derived from procedure SSPHYT02. Includes template updates to current SOP specifications and incorporation of notes into the procedure.
Objective: To standardize a procedure to determine the enzymatic activity of Luciferase and/or to determine the ATP detection limit of Luciferase.
Objective: To standardize a procedure for determining the enzymatic activity of 5’-Nucleotidase.
Objective: To standardize a procedure for the enzymatic assay of β-Glucuronidase.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service