All Photos(1)
About This Item
Empirical Formula (Hill Notation):
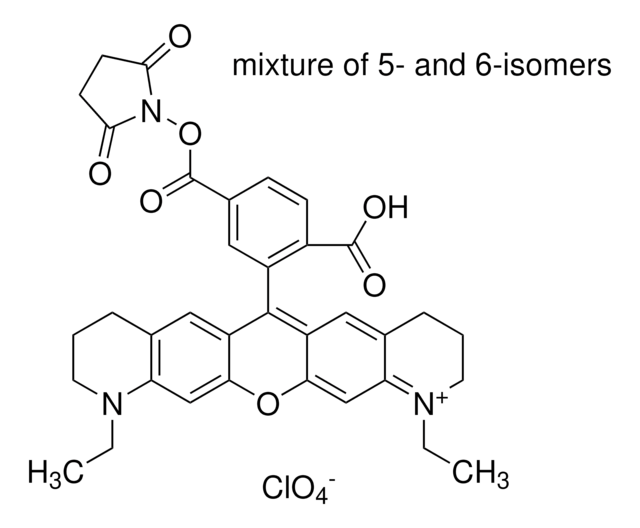
C31H31ClN2O9
Molecular Weight:
611.04
MDL number:
UNSPSC Code:
12352108
NACRES:
NA.32
Recommended Products
product line
BioReagent
Assay
≥90.0% (HPLC)
form
powder
manufacturer/tradename
ATTO-TEC GmbH
λ
in ethanol (with 0.1% trifluoroacetic acid)
UV absorption
λ: 562.0-568.0 nm Amax
suitability
suitable for fluorescence
storage temp.
−20°C
Looking for similar products? Visit Product Comparison Guide
Application
Atto fluorescent labels are designed for high sensitivity applications, including single-molecule detection. Atto labels have rigid structures that do not show any cis-trans isomerization. Thus these labels display exceptional intensity with minimal spectral shift on conjugation.
Atto 565 shows a molar extinction of 120.000 and QY of 92% in water (97% in ethanol). Decay time is 3.4 ns.
Atto 565 shows a molar extinction of 120.000 and QY of 92% in water (97% in ethanol). Decay time is 3.4 ns.
Specific and stable fluorescence labeling of histidine-tagged proteins
Legal Information
This product is for Research use only. In case of intended commercialization, please contact the IP-holder (ATTO-TEC GmbH, Germany) for licensing.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Suman Lata et al.
Journal of the American Chemical Society, 128(7), 2365-2372 (2006-02-16)
Labeling of proteins with fluorescent dyes offers powerful means for monitoring protein interactions in vitro and in live cells. Only a few techniques for noncovalent fluorescence labeling with well-defined localization of the attached dye are currently available. Here, we present
Łukasz Krzemiński et al.
Journal of the American Chemical Society, 133(38), 15085-15093 (2011-08-26)
A combined fluorescence and electrochemical method is described that is used to simultaneously monitor the type-1 copper oxidation state and the nitrite turnover rate of a nitrite reductase (NiR) from Alcaligenes faecalis S-6. The catalytic activity of NiR is measured
Marie-Luise Humpert et al.
Proteomics, 12(12), 1938-1948 (2012-05-25)
PTMs of extracellular domains of membrane proteins can influence antibody binding and give rise to ambivalent results. Best proof of protein expression is the use of complementary methods to provide unequivocal evidence. CXCR7, a member of the atypical chemokine receptor
Takao Nakata et al.
The Journal of cell biology, 194(2), 245-255 (2011-07-20)
Polarized transport in neurons is fundamental for the formation of neuronal circuitry. A motor domain-containing truncated KIF5 (a kinesin-1) recognizes axonal microtubules, which are enriched in EB1 binding sites, and selectively accumulates at the tips of axons. However, it remains
Sebastian van de Linde et al.
Photochemical & photobiological sciences : Official journal of the European Photochemistry Association and the European Society for Photobiology, 8(4), 465-469 (2009-04-02)
We introduce a general approach for multicolor subdiffraction-resolution fluorescence imaging based on photoswitching of standard organic fluorophores. Photoswitching of ordinary fluorophores such as ATTO520, ATTO565, ATTO655, ATTO680, or ATTO700, i.e. the reversible transition from a fluorescent to a nonfluorescent state
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service