Early Microbial Detection and Enumeration with
Milliflex® Rapid System 2.0
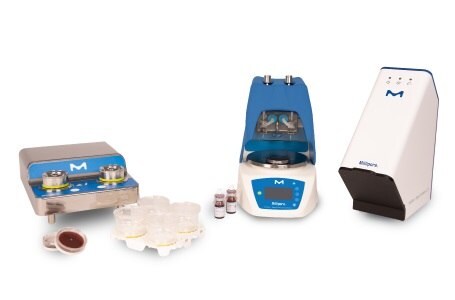
- Milliflex® Rapid System 2.0 for Sterility Testing
- Benefits of Rapid Microbial Detection System
- Rapid Microbiological Methods with Proven Technologies
- Milliflex® Rapid System 2.0: Microbial Testing Workflow
- Regulatory Compliance for the Milliflex® Rapid Method
- Services for Milliflex® Rapid System
- Related Products
Sterility testing is a mandatory microbiology test required by cGMP for pharmaceutical products, medical devices, and other materials, to ensure the products are completely sterile and safe to use. Sterility tests are crucial for quality control and assurance of sterility of a product. Rapid sterility is an alternative test method to the USP Chapter <71>, Ph.Eur. 2.6.1, and JP 4.06 sterility tests that allow for shorter incubation times and faster results as compared to the traditional sterility testing method. The reduction in test time ensures faster release of sterile drug products and quicker corrective actions setting in case of failure.
The Milliflex® Rapid System 2.0 system is an ideal rapid microbial alternative that has proven comparability to traditional methods. The system uses ATP bioluminescence technology for the early detection of viable microorganisms, leaving microorganisms intact for later identification.
Milliflex® Rapid System 2.0 for Sterility Testing
The Milliflex® Rapid System 2.0 is an automated solution for the rapid detection, imaging, and quantification of viable microbial contaminants in filterable samples throughout the manufacturing process.
The system's results will help to improve process control, product yield, and the timely release of final products. In case of contamination, corrective action can be taken earlier, avoiding loss of time, money, and production capacity.
Benefits of Rapid Microbial Detection System
With the Milliflex® Rapid System 2.0 for rapid sterility testing, you can detect microbial contamination in a third of the time of your current method with a sensitivity down to 1 CFU per sample. Traditional sterility tests require an incubation time of at least 14 days to deliver results. By changing the testing approach from liquid media incubation to solid nutrient media, and detection from visual inspection to an automated bioluminescence assay, the Milliflex® Rapid System 2.0 substantially reduces the time to result to only 5 days.
The Milliflex® Rapid System 2.0 offers significant benefits for microbial contamination testing:
- Consistently fast and reproducible results
- Up to four times faster results than membrane filtration or pour plate methods
- Correlation of CFU counts with those of traditional methods
- Data integrity thanks to 21 CFR Part 11 compliant software
- Easy handling and validation
Milliflex® Rapid System Applications
The Milliflex® Rapid System 2.0 is the perfect rapid solution for bioburden and sterility testing needs. It is compatible with a wide range of filterable samples from the pharmaceutical, beverage, and personal care industries:
- Raw materials
- Sterile and non-sterile final products
- Water samples
- Mineral water and beverages such as iced tea, flavored water, beer, and wine
- Cell-based matrices*
*Consult our application scientists for supporting protocols.
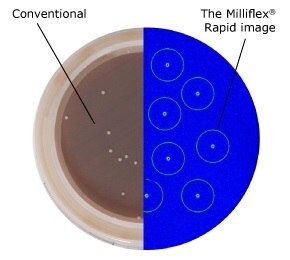
Figure 1.Conventional after 6 days (left) vs. Milliflex® Rapid after 2 days (right) method: C. acnes type III on Schaeddler Blood Agar, 35 °C.
Membrane Filtration
As today’s standard for sample preparation, membrane filtration allows a large volume of product to be processed, and any inhibitory substances are easily rinsed away. The method optimizes the enumeration of microorganisms by filtering fast and ensuring reliable results.
ATP Bioluminescence
Found only in living cells, ATP is a great indicator of cell viability. Unlike other methods that detect both living and dead cells, the ATP method is highly reliable and consistent with the detection rates of current compendial testing methods.
Image Analysis for Enumeration of Microorganisms
The Milliflex® Rapid System 2.0 uses a complementary metal oxide semiconductor (CMOS) camera to detect and enumerate any microcolonies. The ATP concentration required for recognition is equivalent to one yeast or mold cell or approximately 100 bacterial cells, depending on their metabolic state. The camera’s sensitivity, combined with state-of-the-art image analysis and optimized reagents, requires only a short incubation period to generate enough ATP for the detection and enumeration of microcolonies.
Milliflex® Rapid System 2.0: Microbial Testing Workflow
With the proven rapid method Milliflex® Rapid System 2.0 can significantly speed up your sterility and bioburden testing results. Watch the video to learn the many benefits of this automated system along with step-by-step workflow for rapid microbial detection.
The complete workflow for microbial testing including sample preparation, microbial identification, and enumeration of microcolonies is given below.
Step 1
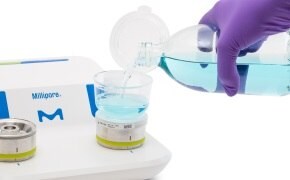
Filter the desired sample volume through a pre-sterilized, ready-to-use disposable Milliflex Oasis® filtration unit according to your standard operating procedure. Transfer the membrane filter onto a Milliflex Oasis® agar plate for incubation. Learn more about the Milliflex Oasis® membrane filtration system.
Step 2

Place the membrane filter on the Milliflex® Rapid 2.0 AutoSpray Station for the application of reagents. The Milliflex® Rapid 2.0 AutoSpray Station automatically applies the two reagents across the membrane surface in order to reveal the ATP present in all living microorganisms.
Step 3

Transfer the membrane filter to the Milliflex® Rapid System 2.0 Detection Tower for enumeration of microcolonies. The system counts each microcolony and stores the data for downloading, printing, and retrieval. Within two minutes, the sample is analyzed, displaying results, along with the batch history.
Regulatory Compliance for the Milliflex® Rapid Method
The ATP bioluminescence technology used by the Milliflex® Rapid System 2.0 is recognized as an alternative detection method by:
- FDA1,2
- PDA Technical Report No. 33: Evaluation, Validation and Implementation of New Microbiological Testing Methods3
Membrane filtration is a methodology recommended by US, European, and Japanese standards to capture microorganisms. The Milliflex® Rapid system 2.0 is calibrated to International light standards [LNE/NIST] and meets electrical conformity to the CE mark.
21 CFR Part 11 Compliance Ready
The Milliflex® Rapid System 2.0 software meets the requirements of FDA Regulation 21 CFR Part 11 for Electronic Records. The software’s powerful batch reporting feature includes capabilities for electronic signing, performing audit trails, and preventing the alteration of data files. Access and editorial privileges are controlled via the software administrator module, ensuring secure data acquisition and audit trails conforming to 21 CFR Part 11. Download the software here.
Services for Milliflex® Rapid System
We provide technical support and a comprehensive service to help you save your QC resources and facilitate the implementation of your Milliflex® Rapid System 2.0 into your daily testing routine. Our highly experienced team of experts is available to assist you with queries regarding the technology, instrument, or any other technical support.
References
To continue reading please sign in or create an account.
Don't Have An Account?