Ni-NTA-Atto Conjugates
Ni-NTA-Atto Conjugates for Sensitive, Specific Detection of Polyhistidine-Tagged Proteins
State-of-the-art recombinant protein applications require reliable methods of detection. Polyhistidine is one of the most popular affinity tags incorporated into recombinant proteins. It can be inserted either at the N- or C-terminus, and expressed in a variety of hosts. Due to its small size, the polyhistidine tag serves as an elegant tool for both protein purification and detection. Immunodetection of His-tagged fusion proteins on a Western blot typically uses an antibody to polyhistidine, followed by a secondary antibody conjugated to an enzyme such as horseradish peroxidase or alkaline phosphatase. The protein is then detected with an appropriate enzyme substrate.
Ni-NTA-Atto conjugates provide specific and highly sensitive detection of His-tagged fusion proteins. The Ni-NTA-Atto complex (Nα,Nα-bis(carboxymethyl)-L-lysine, Nickel(II) complex, conjugated to Atto dye) is specific for polyhistidine tags with minimal crossreactivity. Atto dyes deliver strong fluorescent signals at commonly available wavelengths and with little quenching. Ni-NTA-Atto conjugates can be directly applied either to an SDS-PAGE gel or Western blot membrane for fluorescence imaging, and have been successfully used in living cells.1,2 Detection with Ni-NTA-Atto conjugates requires less incubation time than for protein-antibody binding. No secondary reaction is required, since the Ni-NTA complex is directly conjugated to the fluorophore. This results in a faster and more flexible procedure than the traditional immunodetection method (Figure 1).
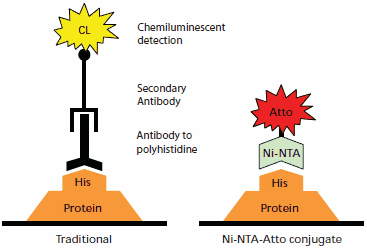
Figure 1.Comparison of protein detection by traditional antibody chemiluminescent immunodetection (left) to Ni-NTA-Atto conjugate detection (right). Immunodetection takes place on a transfer membrane after Western blotting, while the Ni-NTA-Atto conjugates may be used with either transfer membranes or directly on SDS-PAGE gels after fixing.
Direct Detection of Histidine-Tagged Proteins on SDS-PAGE Gels
Direct application of Ni-NTA-Atto conjugates to SDS-PAGE gels provides fast and easy detection for monitoring protein purification or protein expression at different points of time. A detection limit of 50 ng for His-tagged p38 MAPK was observed using fluorescence imaging (Figure 2).
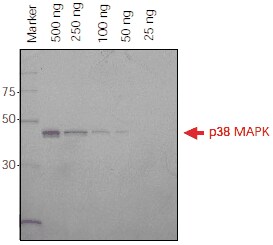
Figure 2.His-tagged p38 MAPK protein (500 ng – 25 ng) was separated on a 4-20% Tris-Glycine SDS-PAGE gel. The gel was fixed overnight in 40% ethanol/10% acetic acid, washed in water and incubated with Ni-NTA-Atto 647N (1:1000) in the dark. The gel was washed and then imaged using a FLA-3000 Fuji® laser scanner with 633 nm excitation and a 675 nm emission filter. Ni-NTA-Atto 647N (λex 647 nm, λem 669 nm) is excited in the red region of the spectrum.
Detection of Histidine-Tagged Proteins after Western Blot Transfer
After electrophoresis, proteins may be transferred from SDS-PAGE gels to low-fluorescence PVDF-membranes, which are more robust to handling. Traditional Western blots work well for polyhistidine tags, but are time consuming. Ni-NTA-Atto conjugates combine the advantages of highly specific detection with a more rapid procedure.
Application of Ni-NTA-Atto conjugates to the membrane yields sensitivity comparable to direct gel detection. Drying the membrane enhances the signal intensity (Figure 3). In addition, the Ni-NTA-Atto conjugates may be stripped from the used membrane with a 60-90 minute incubation in 20 mM EDTA.

Figure 3.His-tagged p38 MAPK protein (500 ng – 25 ng) was separated on a 4-20% SDS-PAGE gel. The protein was transferred to a low-fluorescence PVDF-membrane, blocked overnight with 5% BSA in PBS, rinsed with PBS-T, and incubated with Ni-NTA-Atto 647N (1:1000) in the dark. The membrane was washed and then imaged using a FLA-3000 Fuji laser scanner with 633 nm excitation and a 675 nm emission filter.
Comparison of procedures for the traditional Western immunoblot and Ni-NTA-Atto conjugates is shown in Table 1. Direct SDS-PAGE gel detection is the most convenient as it omits the transfer step. Similar detection limits with Ni-NTA-Atto conjugates are observed for Western blot membranes compared to SDS-PAGE gels, but without risk of gel damage. In either case, the hands-on time is less than for the traditional antibody technique.
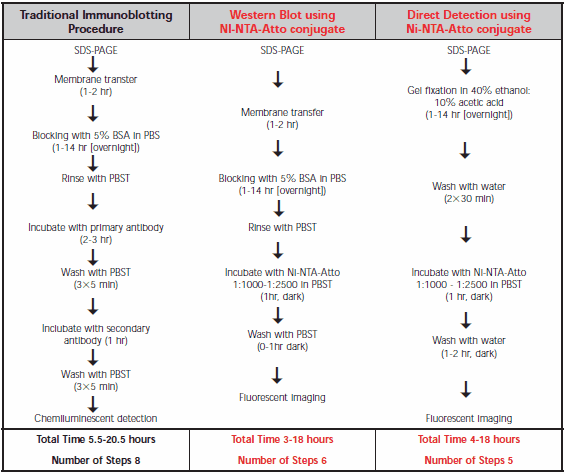
Table 1.Comparison of the protocols for traditional Western immunoblot, Western blot using Ni-NTA-Atto conjugate, and direct detection on an SDS-PAGE gel using Ni-NTA-Atto conjugate. Ni-NTA-Atto stock solution is prepared by dissolving 250 μg in 250 μL PBS.
Ni-NTA-Atto conjugates provide a valuable alternative to traditional antibody immunodetection for specific and sensitive detection of His-tagged fusion proteins. Use of Ni-NTA-Atto reduces experiment time and eliminates time-consuming antibody validation experiments, saving both time and expense.
References
To continue reading please sign in or create an account.
Don't Have An Account?