Ion Pairing for Analysis of Phosphonate Compounds
Phosphonates are a class of compounds commonly used for therapeutic applications of antiviral drugs. These compounds can be difficult to analyze due to their highly polar nature and lack of UV active chromophores. Typical applications involve using ion pair reagents to improve chromatographic selectivity and add functionality for UV or fluorescence response. The concept behind this study was to improve chromatographic retentivity but also increase ionization response for these highly polar analytes using positive ion electrospray ESI+ MS.
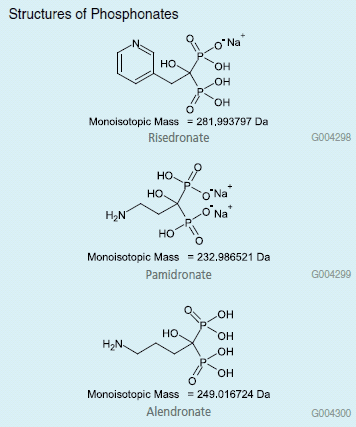
Structures of Phosphonates
In this study N,N-dimethylhexylamine (NNDHA) was chosen to aid in chromatographic selectivity of risedronate, pamidronate and alendronate using traditional reversed phase chromatography. Here the amine functionality of the NNDHA ion pairs with the analyte phosphonate group, resulting in increased hydrophobicity. In addition to increased retention, the reagent adds an increased benefit for mass spectral analysis. NNDHA is also a volatile ion pair reagent and enables adduct formation with the phosphonate functionality, resulting in better ionization in positive ion mode ESI.
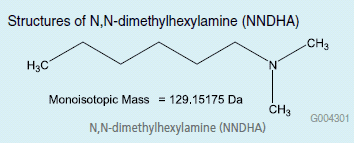
Structures of N,N-dimethylhexylamine (NNDHA)
To prove the concept of increased ionization, first a 1 μg/mL solution of risedronate, pamidronate and alendronate was prepared in 10 mM ammonium acetate 80:20 water:acetonitrile. This solution was then infused into a Thermo LCQ Advantage Ion Trap MS, and a summed spectrum was obtained. Under these conditions none of the phosphonates were detected for the parent ion or any of the sodium or potassium adducts. To improve upon on the ionization, a 10 mM solution of NNDHA in 80:20 water:acetonitrile at pH 5.0 was then prepared and spiked at 1 μg/mL with the phosphonate compounds. Upon infusion of this sample, ions with mass greater than m/z 450 were observed in the spectrum. After calculating the adduct formation, it was determined that observed ions were the multiple NNDHA adducts of the phosphonate analytes. Figure 1 depicts the spectrum from the infusion experiment; the resulting ions at m/z 494, 508 and 542 are the doubly substituted NNDHA adducts of pamidronate, alendronate and risedronate. The NNDHA adduct formation of the phosphonates greatly increased sensitivity of the ion trap MS for these compounds
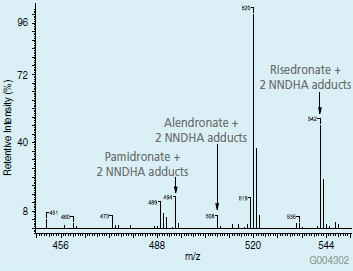
Figure1.Spectrum from ESI+ of phosphonates compounds in solution with NNDHA reagent.
The next step was development of HPLC conditions for the chromatographic separation of these analytes. A 10 mM solution of NNDHA was used to ion pair with the phosphonate compound for increased chromatographic retention. This enabled isocratic separation of risedronate, pamidronate and alendronate in less than 6 minutes using a standard column configuration. In this case, the 1 mL/min column eluent going to the mass spectrometer source was split to 100 μL/min while maintaining good sensitivity of the analytes. The phosphonate standard did not contain the NNDHA; instead the ion pairs were generated in the mobile phase prior to reaching the column inlet.
The use of the NNDHA enabled unique utility within the analysis of these phosphonate compounds, not only was chromatographic retention improved but the added benefit of adduct formation greatly increased detection in the MS instrument. This approach is very similar to traditional derivatization techniques, but is more amenable to modern LC-MS applications. This approach can also be applied to other applications involving difficult acidic compounds with limited retention or limited detection in positive mode electrospray.
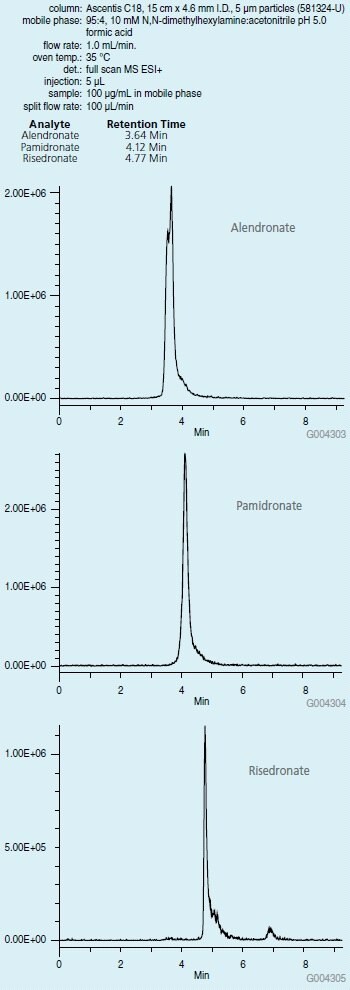
Figure 2.Chromatographic Separation of Phosphonate Compounds on Ascentis C18 (581324-U)
To continue reading please sign in or create an account.
Don't Have An Account?