Using UHPLC/MS (TOF) for Detection of Drugs and Metabolites in Urine Following Optimized Enzymatic Hydrolysis Conditions
Craig Aurand, Manager, Kristen Brown, Pennsylvania State University
Background
β-D-Glucuronide glucuronosohydrolase enzymes, commonly shortened to β-glucuronidase, play an important role in the analysis of biological fluids for the presence of metabolites for drug screening and drug metabolism studies. β-Glucuronidase hydrolyzes glucuronide metabolites back to the native parent drug by the general pathway shown in Figure 1.1 Hydrolysis using β-glucuronidase is often necessary when extensive drug metabolism complicates drug detection in biological samples. For liquid chromatographic analytical methods, the highly-polar glucuronide metabolites that form cannot be easily retained in reversed-phase chromatographic separation, thus hindering the quantitative ability of the analytical method. Hydrolysis of glucuronide metabolites is necessary for gas chromatographic methods. This also commonly includes the addition of secondary derivatization for effective analysis and detection.
The enzyme is ubiquitous throughout living systems because of its important role in carbohydrate metabolism. Although there are numerous β-glucuronidase enzymes available, each enzyme has optimal conditions for hydrolysis of glucuronide metabolites. Variables such as β-glucuronidase enzyme concentration, digestion pH, incubation time, and temperature all play important roles for the effective hydrolysis of glucuronide metabolites.

Figure 1. β-Glucuronidase Hydrolysis Reaction
Goals of the Study
The goal of the study presented here was to determine optimal conditions for β-glucuronidase from various species toward hydrolysis of different compound classes with subsequent analysis by UHPLC/MS (TOF). It will address some of the critical variables that come into play when choosing an appropriate β-glucuronidase enzyme and incubation conditions to perform effective hydrolysis of glucuronide metabolites to the parent drug form. In this study, a set of compounds that are commonly hydrolyzed prior to analysis was used to evaluate the conditions for effective hydrolysis over a range of sources and forms of β-glucuronidase. Various strains of β-glucuronidase enzymes were evaluated. Five drug classes covering opioids, benzodiazepines, steroids, cannabinoids, and pain management drugs and eleven β-glucuronidases that differ in source, type, or formulation were included in the study (Tables 1 and 2).
Experimental
Preparation of Glucuronide Standards and Spiked Urine Samples
A stock solution of 10 µg/mL of each glucuronide metabolite was prepared in water. Human urine was spiked at 200 ng/mL with each glucuronide metabolite.
Preparation of β-Glucuronidase Solutions
Lyophilized forms of β-glucuronidase were prepared in the following manner: Stock solutions of 50 kU/mL of each β-glucuronidase were prepared in a 100 mM ammonium acetate buffer at pH 5.0 and 6.0. Solutions were kept at 4 °C when not in use. Liquid forms were used as supplied.
Sample Digestion with β-Glucuronidase
Spiked stock urine samples were prepared at 10 mL volumes for multiple replicate sampling. Urine samples were spiked at 200 ng/mL with all glucuronide metabolites of test compounds. To the 10 mL urine samples, 2 mL of each corresponding pH buffer was added followed by 2 mL of β-glucuronidase solution. The resultant concentration of β-glucuronidase was 10 kU/mL per urine sample.
Urine samples were then digested at 60 °C in a convection oven for 120 minutes. Samples were removed from the oven and allowed to cool. A 1.4 mL aliquot was removed for further sample preparation using solid-phase extraction (SPE) followed by analysis by UHPLC/MS (TOF).
Hydrolysis Variables
As part of this optimization study, experiments were conducted to evaluate the impact of incubation time, incubation temperature, pH, and enzyme concentration on the conversion of glucuronide metabolites to their corresponding parent drugs. Although these conditions are an important portion of the enzymatic conversion, full experimental details are beyond the scope of this report. The range of incubation times, temperatures, pH, and enzyme concentration tested are as follows:
- Incubation time: 30 minutes, 60 minutes, 120 minutes
- Incubation temperature: 45 °C, 55 °C, 60 °C, 65 °C
- Incubation pH: 4.5, 5, 6
- β-Glucuronidase concentration: 10 kU/mL and 50 kU/mL
Incubation Time
A rapid conversion of the glucuronide metabolite to the parent drug was observed for the short 30 minute incubation time for a majority of the enzymes tested. The conversion was optimal at the 120 minute incubation time for all drug/β-glucuronidase combinations. Subsequent experiments were standardized on a 120 minute incubation time.
Incubation Temperature and Enzyme Concentration
Incubation temperature played an important role in enzymatic activity. The role of β-glucuronidase enzyme concentration was not as significant a rate-limiting step as time or temperature. A significant increase in conversion was not observed between the 10 kU/mL and 50 kU/mL at the 120 minute incubation time study. The 10 kU/mL enzyme concentration was the lowest level incorporated. In the case of incubation temperatures above 65 °C, there was a noted decrease in glucuronide conversion with the 50 kU/mL enzyme concentration. Further study centered on 10 kU/mL enzyme concentration.
Incubation pH
Of all the digestion conditions evaluated, incubation pH had the most significant impact on effectiveness of β-glucuronidase activity. Because pH had such a major impact, the bulk of the results reported in this study are focused around the role of sample pH and the effectiveness of the various β-glucuronidase enzymes. The experimental conditions for pH studies were as follows:
- Incubation time: 120 minutes
- Incubation temperature: 60 °C
- β-Glucuronidase concentration: 10 kU/mL
- Incubation pH: 4.5, 5.0, 6.0
- Analyte concentration in urine: 200 ng/mL
Solid Phase Extraction (SPE) Method
SPE removes excess enzyme that can interfere with the analysis and foul the HPLC columns. Samples were processed by SPE using a mixed mode cation exchange/hydrophobic stationary phase. Native (parent) drugs were eluted from the SPE tube using a basic acetonitrile elution solvent. All sample processing was conducted in replicate (n=4).
- SPE tube/cartridge: Supel™-Select SCX, 60 mg/3 mL (Product No. 54241-U)
- Conditioning step: 1 mL 1% formic acid in acetonitrile followed by 1 mL water
- Sample loading: 1.4 mL spiked and hydrolyzed urine
- Washing step: 2 mL water followed by 1 mL 25% (v/v) methanol in water
- Elution: 1 mL 10% (w/v) ammonium hydroxide in acetonitrile
- Sample post-treatment: evaporate under nitrogen and reconstitute in 1 mL water
UHPLC/MS (TOF) Conditions
All analyses were conducted using gradient elution on a Titan™ C18 1.9 µm UHPLC column. Glucuronide metabolite standards of the class compounds were spiked into control urine samples for determination of hydrolysis conversion. This known concentration of glucuronide metabolite in the urine sample was then used to calculate conversion to the parent drug. Standard solutions of native parent drug (10–300 ng/mL in water) were used to develop calibration curves on the UHPLC MS/TOF system. Chromatographic conditions appear in Figure 2.
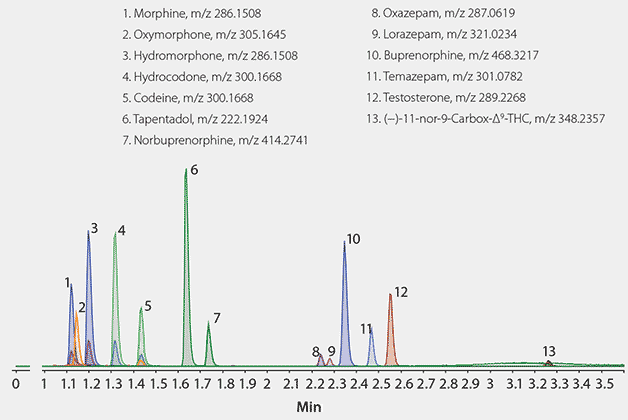
Figure 2. LC/MS (TOF) Analysis of Drugs and Their Glucuronide Metabolites on Titan C18 after Solid Phase Extraction (SPE) using Supel-Select SCX following β-Glucuronidase Enzyme Digestion
Conditions
Column: Titan C18, 5 cm ~ 2.1 mm I.D., 1.9 µm particles (Product No. 577122-U); mobile phase: [A] 5 mM ammonium formate; [B] 5 mM ammonium formate in acetonitrile:water (95:5); gradient: 5 to 90% B in 2.5 min, held for 0.5 min, to 5% B in 0.1 min, held for 2 minutes; flow rate: 0.3 mL/min; column temp: 35 °C; detector: MS, ESI(+), full scan, m/z 100-1000; injection: 2 µL; sample: urine spiked with drug glucuronide metabolites, hydrolyzed, and extracted via SPE as described in the text, final concentration 100 ng/mL; system: Agilent 1290 Infinity with Agilent 6210 TOF
Results
The optimized enzyme hydrolysis conditions for each drug are summarized in Table 3. A chromatogram of the test compounds hydrolyzed and extracted from urine, separated on the Titan C18 column, and analyzed by LC/MS (TOF) is shown in Figure 2.
The critical pair of codeine and hydrocodone had sufficient resolution under these gradient conditions. This was extremely important since these compounds are isobaric. No sample matrix interference was observed which demonstrates the effectiveness of the sample preparation method.
Effect of pH on Hydrolysis by β-Glucuronidase
The pH used during the digestion was the most significant factor of all design variables. For example, Figure 3 shows glucuronide hydrolysis under the three pH conditions using the E. coli Type IX-A enzyme. Only marginal hydrolysis occurred at pH 4.5 for all compound classes, but pH 5 and pH 6 had significant hydrolysis. Hydrolysis above pH 5 was dependent on the compound class. The benzodiazepines, pain management, and steroid test compounds had substantial conversion above pH 5, while the opioids had only marginal conversion across any pH value. This would indicate the E. coli Type IX-A enzyme would not be suitable as a general hydrolysis for glucuronide metabolites but may be suitable for class-specific compounds.

Figure 3.Escherichia coli β-Glucuronidase Type IX-4 (Product No. G7396): Effect of pH on Hydrolysis Efficiency
In comparison, the effect of pH on limpet (Patella vulgata) β-glucuronidase Type L-II hydrolysis is shown in Figure 4. Hydrolysis was observed for all classes of compounds across all incubation pH values. Optimal hydrolysis for all classes was observed at pH 5, but this was not necessarily the optimal pH for each individual class. The benzodiazepines had optimal hydrolysis at pH 5 while the opioids had optimal hydrolysis at pH 6. These results would suggest the Patella vulgata Type L-II β-glucuronidase may be suitable as a general hydrolysis enzyme independent of compound class.

Figure 4. Limpet (Patella vulgata) β-Glucuronidase Type L-II (Product No. G8132): Effect of pH on Hydrolysis Efficiency
The experimental results summarized in Table 3 present the optimal hydrolysis conditions for the specific compounds of each compound class. These results exhibited the highest level of hydrolysis for each specific compound, but may not be the optimal conditions for the entire class. In the case of benzodiazepine glucuronides, the E. coli enzyme did give optimal conversion for all benzodiazepines tested. In the case of opioids, the hydrolysis conditions varied depending on the opioid glucuronide. In this case, the combination of β-glucuronidase/compound was much more compound specific than class general. The E. coli β-glucuronidase gave the best degree of conversion for the steroid and cannabinoid test analytes.
Conclusions
To optimize hydrolysis using β-glucuronidase, factors such as incubation time, temperature, hydrolysis pH, enzyme source, and enzyme concentration must be evaluated for each glucuronide metabolite to be analyzed. In the study reported here, the hydrolysis pH was determined to be the most important factor for effective hydrolysis of glucuronide metabolites in urine. In some cases, a specific β-glucuronidase enzyme was effective for hydrolysis across a class of glucuronide metabolites. However, in most cases optimal hydrolysis conditions were compound dependent.
For a general-purpose enzyme, the aqueous solution of β-glucuronidase from limpets (Patella vulgata), product number G2174, gave the best overall conversion for all classes of compounds included in this study across all the pH conditions. This was not necessarily the optimal hydrolysis for each class or specific compound, but the limpet β-glucuronidase produced sufficient hydrolysis for all classes regardless of hydrolysis pH. For optimal hydrolysis at specific β-glucuronidase and hydrolysis pH, the best conditions for the overall conversion of all classes of compounds within this study were observed with the pH 5 conditions of the β-glucuronidase from bovine liver Type B-1, product number G0251.
References
To continue reading please sign in or create an account.
Don't Have An Account?