LC/MS Analysis of Bile Acids and Their Conjugates on Ascentis® Express C18
Materials
analytical column
mobile phase component
standard
CONDITIONS
sample/matrix
Plasma proteins were removed with addition of 900 μL of acetonitrile, containing deuterated internal standards, to 250 μL of human EDTA plasma. The mixture was vortexed, (centrifuged and the supermatant evaporated before being reconstituted in a 50:50 solution of methanol and water. 10 μL (corresponding to 8.57 μL of plasma) was injected into the HPLC.)
column
Ascentis Express C18, 15 cm x 4.6 mm I.D., 2.7 μm particles (53829-U)
mobile phase
[A]: Water (5 mM ammonium acetate and 0.012% formic acid); [B]: Methanol (5 mM ammonium acetate and 0.012% formic acid)
gradient
70 to 95% B in 10 minutes held 4 minutes
flow rate
0.6 mL/min
column temp.
40 °C
detector
ESI(-), MRM Mode
Description
Analysis Note
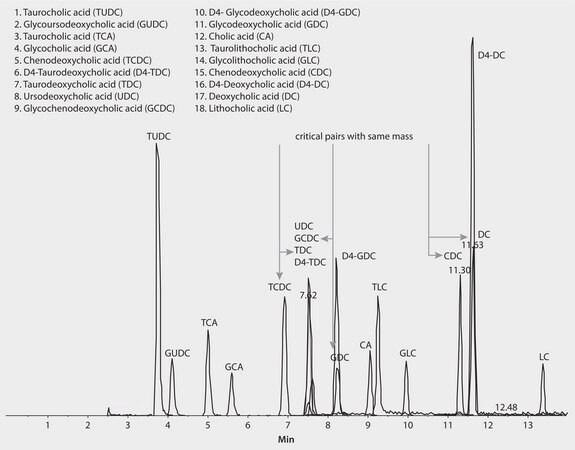
A fast robust LC-MS/MS method was developed on a C18 Ascentis Express Fused-CoreR Particle Column allowing the 15 bile acid species to be measured individually rather than the alternative measurement of a total concentration by colourimetric kinetic enzyme assays.
Legal Information
Ascentis is a registered trademark of Merck KGaA, Darmstadt, Germany