All Photos(1)
About This Item
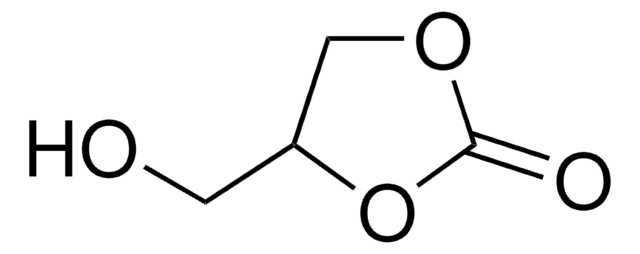
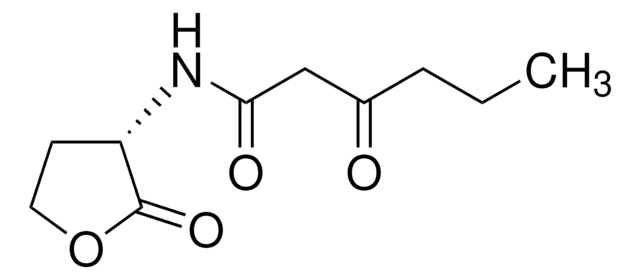
Empirical Formula (Hill Notation):
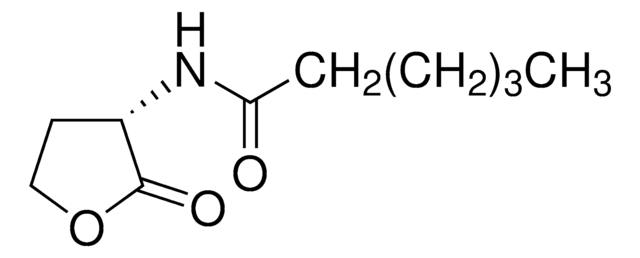
C11H19NO3
CAS Number:
Molecular Weight:
213.27
MDL number:
UNSPSC Code:
12352209
eCl@ss:
32160406
PubChem Substance ID:
NACRES:
NA.26
Recommended Products
product name
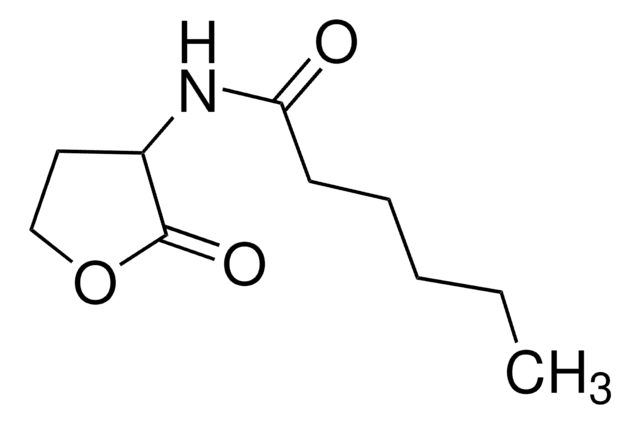
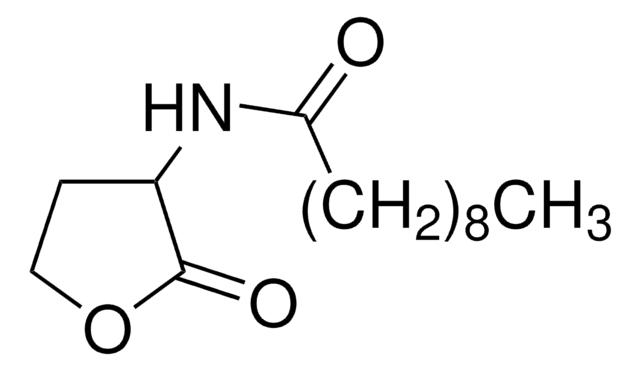
N-Heptanoyl-DL-homoserine lactone, ≥97.0% (HPLC)
Assay
≥97.0% (HPLC)
form
powder with small lumps
color
white to faintly brown
storage temp.
−20°C
SMILES string
CCCCCCC(=O)NC1CCOC1=O
InChI
1S/C11H19NO3/c1-2-3-4-5-6-10(13)12-9-7-8-15-11(9)14/h9H,2-8H2,1H3,(H,12,13)
InChI key
FTMZLSDESAOPSZ-UHFFFAOYSA-N
Application
Application test: Induces violacein expression in a Chromobacterium violaceum mutant usually not able to produce homoserine lactones.
Biochem/physiol Actions
N-Heptanoyl-DL-homoserine lactone (C7HSL) is among a group of homoserine lactones that includes; N-octanoyl-homoserine lactone (N-C8-HSL), N-Decanoyl-DL-homoserine lactone (N-C10-HSL), N-(3-oxodecanoyl) homoserine-L-lactone (3-oxo-C10 HSL), N-(3-oxododecanoyl)homoserine-L-lactone (3-oxo-C12-HSL), N-(3-Oxotetradecanoyl)-L-homoserine lactone (3-oxo-C14-HSL, N-(3-hydroxydecanoyl)-L-homoserine lactone, and N-(3-hydroxyoctanoyl)-L-homoserine lactone involved in the processes of bacterial quorum sensing. These N-acyl-homoserine lactones are used to study the processes and mechanisms of bacterial quorum sensing.
N-Heptanoyl-DL-homoserine lactone is a member of N-acyl-homoserine lactone family. N-acylhomoserine lactones (AHL) regulate gene expression in gram-negative bacteria, such asEcherichia and Salmonella are involved in quorum sensing, cell to cell communication among bacteria. Some AHLs are potent chemoattractants for human immune cells such as neutrophils.
Packaging
Bottomless glass bottle. Contents are inside inserted fused cone.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Detection of acyl-homoserine lactones by Escherichia and Salmonella.
Soares JA, Ahmer BM.
Current Opinion in Microbiology, 12, 188-193 (2011)
Armando M Pomini et al.
Journal of natural products, 71(6), 1032-1036 (2008-05-10)
(S)-N-Heptanoylhomoserine lactone is an uncommon acyl odd-chain natural product employed by many Gram-negative bacteria as a signaling substance in chemical communication mechanisms known as quorum sensing. The absolute configuration determination of the metabolite produced by the phytopathogen Pantoea ananatis Serrano
Ali E McClean et al.
Phytopathology, 102(2), 195-203 (2012-01-13)
Several members of the bacterial genus Brenneria are pathogenic on different tree species. Cell-free extracts from the bacterial phytopathogens Brenneria rubrifaciens, B. salicis, and B. nigrifluens induced production of the red pigment rubrifacine in the B. rubrifaciens bruI insertional mutant
Frank Wilco Bartels et al.
Biophysical journal, 92(12), 4391-4400 (2007-03-27)
Intercellular communication by means of small signal molecules coordinates gene expression among bacteria. This population density-dependent regulation is known as quorum sensing. The symbiotic nitrogen-fixing bacterium Sinorhizobium meliloti Rm1021 possesses the Sin quorum sensing system based on N-acyl homoserine lactones
Selvaraj Poonguzhali et al.
Journal of microbiology and biotechnology, 17(2), 226-233 (2007-12-07)
Members of Methylobacterium, referred as pink-pigmented facultative methylotrophic bacteria, are frequently associated with terrestrial and aquatic plants, tending to form aggregates on the phyllosphere. We report here that the production of autoinducer molecules involved in the cell-to-cell signaling process, which
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service