745723
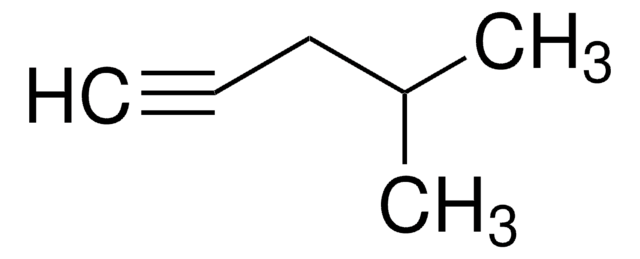
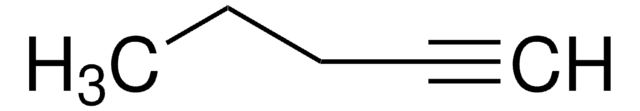
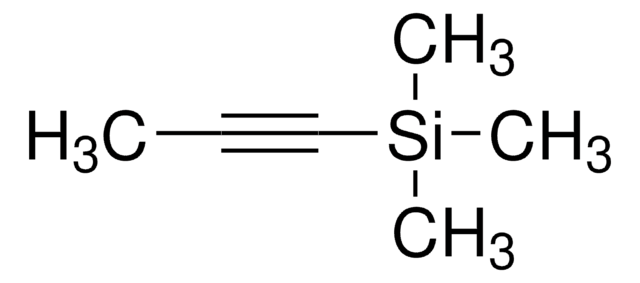
3-Methyl-1-butyne
95%
Synonym(s):
1-Isopentyne, 2-Methyl-3-butyne, 3,3-Dimethyl-1-propyne, Isopropylacetylene, Isopropylethyne
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H8
CAS Number:
Molecular Weight:
68.12
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
95%
form
liquid
refractive index
n20/D 1.374
density
0.666 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
C#CC(C)C
InChI
1S/C5H8/c1-4-5(2)3/h1,5H,2-3H3
InChI key
USCSRAJGJYMJFZ-UHFFFAOYSA-N
Application
3-Methyl-1-butyne can be used as a reactant in the ruthenium-catalyzed synthesis of functionalized cycloheptadienes and titanium-catalyzed [2+2+1] multicomponent pyrrole synthesis. It is also used as a precursor in the total syntheses of (+)-frondosin A, (−)-citrinadin A, (+)-citrinadin B and coraxeniolide A.
3-Methyl-1-butyne is a terminal alkyne that can be used to prepare:
- Maleimide-fused cyclopentenones by reacting with N-substituted maleimides via Co2(CO)8-mediated Pauson−Khand reaction.
- An alkyne carboxylic acid (Alkyne Segment C) intermediate, which is used in the total synthesis of oscillatoxin D and its analogs.
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Enantioselective total synthesis of (-)-citrinadin A and revision of its stereochemical structure.
Bian, Zhiguo and Marvin, Christopher C and Martin, Stephen F
Journal of the American Chemical Society, 135(30), 10886-10889 (2013)
Synthetic Route to Oscillatoxin D and Its Analogues
Nokura Y, et al.
Organic Letters, 19(21), 5992-5995 (2017)
Total synthesis of (+)-frondosin A. Application of the Ru-catalyzed [5+ 2] cycloaddition.
Trost, Barry M and Hu, Yimin and Horne, Daniel B
Journal of the American Chemical Society, 129(38), 11781-11790 (2007)
Metal carbene-promoted sequential transformations for the enantioselective synthesis of highly functionalized cycloheptadienes.
Deng L, et al.
Journal of the American Chemical Society, 127(5), 1342-1343 (2005)
An unconventional approach to the enantioselective synthesis of caryophylloids.
Larionov, Oleg V and Corey, EJ
Journal of the American Chemical Society, 130(10), 2954-2955 (2008)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service