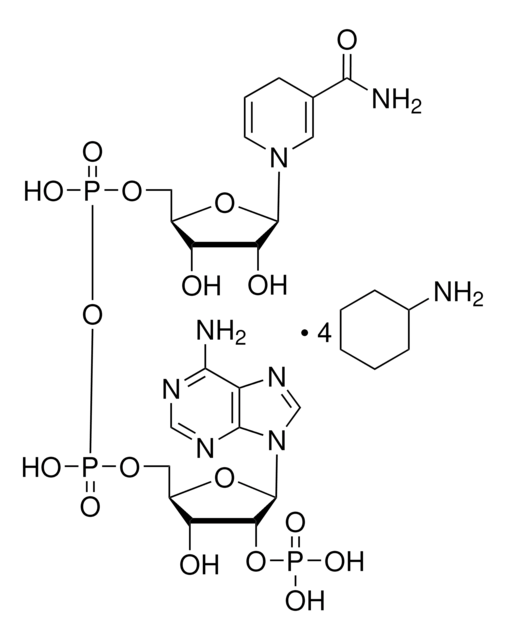
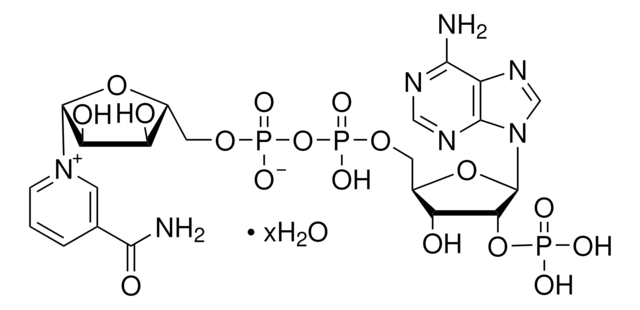
N7505
β-Nicotinamide adenine dinucleotide 2′-phosphate reduced tetrasodium salt hydrate
≥97% (HPLC)
Synonym(s):
β-NADPH, 2′-NADPH hydrate, Coenzyme II reduced tetrasodium salt, Dihydronicotinamide adenine dinucleotide phosphate tetrasodium salt, NADPH Na4, TPNH2 Na4, Triphosphopyridine nucleotide reduced tetrasodium salt
About This Item
Recommended Products
Assay
≥97% (HPLC)
≥97% (spectrophotometric assay)
form
powder
solubility
10 mM NaOH: soluble 50 mg/mL, clear
storage temp.
−20°C
SMILES string
[Na+].[Na+].[Na+].[Na+].NC(=O)C1=CN(C=CC1)[C@H]2O[C@@H](COP([O-])(=O)OP([O-])(=O)OC[C@H]3O[C@H]([C@H](OP([O-])([O-])=O)[C@@H]3O)n4cnc5c(N)ncnc45)[C@H](O)[C@@H]2O
InChI
1S/C21H30N7O17P3.4Na/c22-17-12-19(25-7-24-17)28(8-26-12)21-16(44-46(33,34)35)14(30)11(43-21)6-41-48(38,39)45-47(36,37)40-5-10-13(29)15(31)20(42-10)27-3-1-2-9(4-27)18(23)32;;;;/h1,3-4,7-8,10-11,13-16,20-21,29-31H,2,5-6H2,(H2,23,32)(H,36,37)(H,38,39)(H2,22,24,25)(H2,33,34,35);;;;/q;4*+1/p-4/t10-,11+,13-,14+,15-,16+,20-,21+;;;;/m0..../s1
InChI key
WYWWVJHQDVCHKF-MPUNMZHWSA-J
Looking for similar products? Visit Product Comparison Guide
Biochem/physiol Actions
Preparation Note
Other Notes
also commonly purchased with this product
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Neoplastic cells are highly dependent on the de novo synthesis of nucleotides to maintain sufficient pools to support DNA replication and the production of RNA.
Information on fatty acid synthesis and metabolism in cancer cells. Learn how proliferatively active cells require fatty acids for functions such as membrane generation, protein modification, and bioenergetic requirements. These fatty acids are derived either from dietary sources or are synthesized by the cell.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service