MC-Media Pad – Validation Summary
A Convenient and Approved Method for Counting Contaminants in Food and Beverage Products
The MC-Media Pad is a convenient method for rapid routine testing of raw materials and in-process testing of finished food and beverage products for microbial contamination.
The MC-Media Pad solution is composed of a series of ready-to-use pads for total count and specific detection and enumeration of indicator organisms. MC-Media Pads target 4 key applications in food and beverage microbial testing (Table 1). The MC-Media Pad method has been independently tested and evaluated. Media Pad Rapid Aerobic Count (RAC), MC-Media Pad Coliform, MC-Media Pad E. coli & Coliform and MC-Media Pad Yeast & Mold received certification from AOAC Research Institute as a Performance Tested Method℠(1, 2, 3, 4).
This document will focus on results obtained for the 4 certified MC-Media Pads: MC-Media Pad RAC, MC-Media Pad Coliform, MC-Media Pad E. coli & Coliform and MC-Media Pad Yeast & Mold.
Technology and Principle
MC-Media Pad technology is based on a fabric pad placed on an adhesive support and covered with a transparent gas permeable lid. The fabric pad is a multi-layered structure composed of a water-soluble high polymer laminated with porous non-woven fabric. Dedicated formulations of culture media incorporating nutrients and selective agents are coated onto the fabric pad. The MC-Media Pad RAC promotes the growth of aerobic bacteria in red colonies. The MC-Media Pad Coliform nutrient film also contains X-Gal (5-bromo-4-chloro-3-indoxyl-βD-galactopyranoside) which is hydrolyzed by β-galactosidase in coliforms to produce blue colonies. The MC-Media Pad E. coli & Coliform, in addition to X-Gal, contains Salmoglucuronic acid (6-chloro-3-indoxyl-βD-glucuronic acid) which is hydrolyzed by β-glucuronidase in E. coli to specifically stained E. coli colonies purple. MC-Media Pad Yeast & Mold promotes the growth of yeasts and molds in red colonies.
The fabric pad has been specifically developed for the testing of 1 mL food samples. Sample solution diffuses throughout the entire pad to dissolve and release the nutrient compound forming a highly viscous solution.
The combination of the coated pad and the gas permeable lid promotes the selective or universal growth of microorganisms and enables enumeration. MC-Media Pad RAC, MC-Media Pad Coliform, MC-Media Pad E. coli & Coliform and MC-Media Pad Yeast & Mold performances have been challenged with a range of relevant food matrices either naturally or artificially contaminated with indicator organisms.
Method
Sample Preparation
Portions of 50 g, 25 g or 25 mL of each food sample were added to a stomacher bag or blender cup. 450 mL or 225 mL of the relevant diluent (Peptone water, Butterfield’s phosphate buffer, sterile physiological saline or sodium citrate) was then added to the stomacher bag or blender cup and stomached or homogenized for 2 min. Decimal dilutions can be performed depending on the sample.

Figure 1.MC-Media Pad Protocol
Results
MC-Media Pad RAC
Total aerobic enumeration has been determined for the 10 following food matrices: Cooked, peeled, chilled cold water prawns (1.3% salt); prewashed bagged flat leaf parsley; chilled tuna pate (23% fat, 0.9% salt); fermented strawberry yogurt drink (1.8% fat containing Lactobacillus acidophilus and Bifidobacterium and Lactobacillus casei); fresh raw chicken breast fillets (2% fat); fresh raw lean pork mince (5% fat); cold press green vegetable juice (cucumber, celery, spinach, lemon, lettuce, pear, pineapple); full fat soft cream cheese (24% fat), fresh chilled egg mayonnaise sandwish on white sliced bread (5% fat, 0.9% salt) and fresh feta cheese deli pasta salad (3.4% fat, 0.5% salt).

Figure 2.All grown colonies develop reddish color
Rapid detection incubation (35 °C, 24 h) and standard detection incubation (35 °C, 48 h) conditions were used. The standard incubation condition is applicable for all food matrices but is more appropriate when food samples contain psychrophilic bacteria or a large amount of acidic bacteria since they tend to grow slowly at non-optimum temperatures. Among the 10 food matrices, 7 were selected to compare the performance in the rapid detection incubation conditionversus the reference method.
Total aerobic bacteria was detected after 24 hours of incubation at 35 °C for the 7 matrices and after 48h at 35 °C for the 10 matrices.
In a separate study, all matrices were compared to the ISO 4833:2013 method with an incubation of 72 h at 30 °C. Total aerobic bacteria was detected after 72 h of incubation at 30 °C for the 10 matrices.
All grown colonies develop reddish color
Regulatory Approval
MC-Media Pad RAC was certified by AOAC Research Institute as a Performance Tested MethodsSM Program. Certificate number is 091702.
MC-Media Pad Coliform
Inclusivity and Exclusivity Tests
Inclusivity and exclusivity tests have been performed on 48 coliform and 39 non-coliform type strains including some food isolates.1 BGLB broth was used as control. MC-Media Pad Coliform and BGLB broth were incubated 24 h at 35 °C.
All coliform strains on MC-Media Pad showed positive results with a blue-green staining of each colony. The results indicate an inclusivity rate of 100%.1
Results for all non-coliform strains were negative both in the BGLB and MC-Media Pad Coliform indicating an exclusivity rate of 100%.1

Figure 3.Coliforms develop blue/blue-green colored colonies due to β-galactosidase production
Food Matrices Tested
Detection of coliforms in 26 food samples have been performed. Food types belong to 9 food categories: meat, poultry, fish and seafood, fruits and vegetable,dairy, chocolate and bakery, animal feeds, pasta.
Results showed a detection of coliforms after 24 hours of incubation at 35 °C.1
Coliforms develop blue/blue-green colored colonies due to β-galactosidase production. Gram negative noncoliform bacteria form colorless colonies.
Regulatory Approval
MC-Media Pad Coliform was certified by AOAC Research Institute as a Performance Tested Methods℠ Program.
The certificate number is 100402.
MC-Media Pad E. coli & Coliform
Inclusivity and Exclusivity
Inclusivity and exclusivity tests have been performed on 24 strain types of E. coli, 8 E. coli strains isolated from foods, 21 coliform strain types other than E. coli, and 12 coliform strains from food 2, and 65 non-coliform strains were added in the study. BGLB broth was used as the control. MC-Media Pad E. coli & Coliform and BGLB broth were incubated 24 h at 35 °C.
Strains showing gas production in BGLB grew as coliform blue colonies on MC-Media Pad E. coli & Coliform. All E. coli strains produced a purple to indigo staining on MC-Media Pad. The results indicate an inclusivity rate of 100%.2

Figure 4.Coliforms develop blue/blue-green colored colonies due to β-galactosidase production
Results of all non-coliform strains were negative both in the BGLB and MC-Media Pad E. coli & Coliform except Aeromonas hydrophila, Serratia marcescens and Serratia rubidae which showed a positive result on MC-Media Pad indicating an exclusivity rate of 95.4%.2
Food Matrices Tested
Detection of coliforms and E. coli has been performed on 100 samples of meats and meat products.
Results showed a detection of coliforms and E. coli after 24 hours of incubation at 35 °C.
Coliforms develop blue/blue-green colored colonies due to β-galactosidase production whereas E. coli form indigo to purple colored colonies due to specific β-glucuronidase for E. coli. Gram-negative non-coliform bacteria form colorless colonies.
Regulatory Approval
MC-Media Pad E. coli & Coliform was certified by AOAC Research Institute as a Performance Tested Methods℠Program.
The certificate number is 070901.
MC-Media Pad Yeast & Mold
Comparison of MC-Media Pad Yeast and Mold and FDA/BAM Reference Method
Yeast and mold detection has been performed on contaminated food and artificially contaminated samples, specifically orange juice concentrate, cake mix, breaded chicken nuggets, dry pet food and yogurt.
Products were inoculated at 3 inoculation levels: low (102 CFU/g), medium (103 CFU/g) and high (104 CFU/g).
DG18 agar was used a control for foods with high water activity (aw>0.95), and DRBC agar was used as control for low water activity food samples (aw<0.95).
Results showed a detection of yeasts and molds after 48 to 72 hours of incubation at 25 °C.3
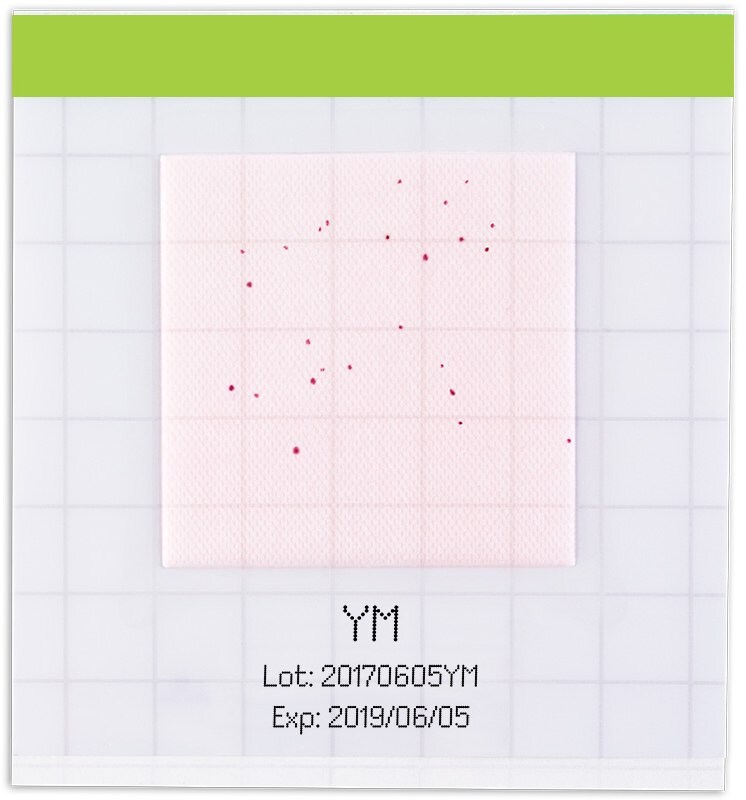
Figure 5.All grown colonies develop a reddish color
All grown colonies develop a reddish color. Yeasts appear as circular reddish colored colonies, whereas molds appear as diffuse and fuzzy round, reddish colored colonies.
Regulatory Approval
MC-Media Pad Yeast & Mold was certified by AOAC Research Institute as a Performance Tested Methods℠ Program.
The certificate number is 111401.
Conclusion
This application study shows that the AOAC certified MC-Media Pad RAC, MC-Media Pad Coliform, MC-Media Pad E. coli and Coliform and MC-Media Pad Yeast and Mold can be used to detect and easily enumerate indicator organism contamination in a broad range of food and beverage matrices.
Chromogenic indicators and dedicated culture media produce specific results and allow ease of interpretation. Additionally providing the benefits of a convenient media method through a well-defined inoculation area and reduction of required space for storage, incubation and waste.
References
Para seguir leyendo, inicie sesión o cree una cuenta.
¿No tiene una cuenta?