Sodium Triacetoxyborohydride
Amine synthesis is one of the most common organic transformations when designing new drug candidates, and the reductive amination of carbonyl compounds is among the most useful and important tools to achieving structurally diverse primary, secondary, and tertiary amines. Sodium triacetoxyborohydride (NaBH(OAc)3) is particularly effective in reductive aminations due to its large scope, mildness, and selectivity.1 It is preferred to sodium cyanoborohydride (NaBH3CN) in many applications due to reduced toxicity of the side products formed, and better yields and reproducibility during synthesis. A typical reaction is taken from the synthesis of a key intermediate of a potent histamine H3 receptor antagonist (Scheme 1).2

Scheme 1.Receptor Antagonist
The reductive aminations of complex substrates also proceed smoothly using sodium triacetoxyborohydride, as in the example shown in Scheme 2. The product is an intermediate in a ring closing metathesis approach to a pentaheterocyclic ring system.3
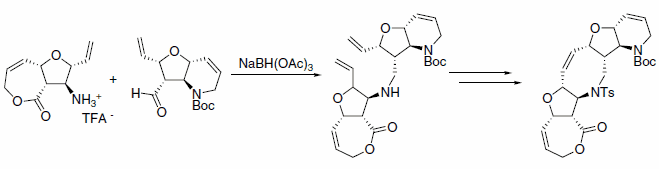
Scheme 2.Triacetoxyborohydride
A unique, one-pot synthesis of substituted N-acylpiperazinones from N-(2-oxoethyl)amides and α-amino esters by a novel tandem reductive amination-transamidation-cyclization process has been described (Scheme 3). This protocol was applied to the synthesis of a conformationally constrained farnesyltransferase inhibitor.4
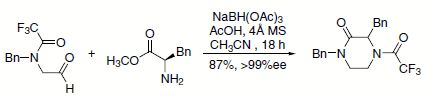
Scheme 3.Farmesyltransferase-inhibitor
A recent report detailed a one-pot, three-component reaction to produce 1,2-disubstituted-3-alkylidenylpyrrolidines using sodium triacetoxyborohydride for in situ reduction of the intermediate pyrrolium salts (Scheme 4). In these reactions, only the (E)-isomer of the product is generated.5
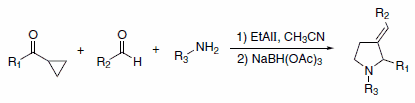
Scheme 4.Pyrrolium salts
Recently, sodium triacetoxyborohydride was used to stereoselec-tively reduce 4-ketoprolines to the corresponding trans-hydroxy-proline in excellent yields (Scheme 5). By comparison, reduction of the 4-ketoproline esters failed to provide any product.6
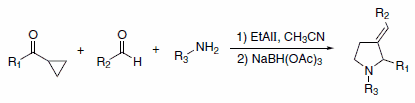
Scheme 5.Ketoprolines
References
Para seguir leyendo, inicie sesión o cree una cuenta.
¿No tiene una cuenta?