Key Steps in Flow Cytometry Protocols
Minor details in the key steps of a flow cytometry assay can have major impacts on the resulting data. There are four steps in most flow cytometry protocols:
Sample Preparation
Any cell population that can be made into a single cell suspension can be assessed by flow cytometry. The following three points are important to consider:
- To prepare an ex vivo cell population, freshly dissected tissue is often gently homogenized using mechanical dissociation methods, and different cell types are separated via density gradient centrifugation to remove intracellular matrix material, debris, and irrelevant cell populations.
- It is important to note that adherent cells must be detached from cell culture vessel surfaces using enzymatic solutions or calcium chelation reagents prior to use.
- Suspended cell cultures only need to be counted and assessed for viability.
Cell counts/titers refer to viable cells in suspension that can be determined by using an automated cell counter and applying gating to exclude dead cells/debris. Alternatively, viability can be assessed by microscope-aided counting of the number of live cells in a known volume, such as by using a hemocytometer and Trypan blue dye, which is excluded by the intact membrane of live cells, and only the non-viable dead cells uptake this dye.
Blocking
To prevent nonspecific binding of primary antibody(ies) to suspended cells, an anti-Fc antibody dilution (specific to the sample species) may be applied. This prevents binding of the Fc or constant region of the antibody by Fc receptors, which are present on most cell types. Fc block is typically added to washed cells in a small volume. The staining antibody dilution is added immediately at the end of the blocking incubation without a wash step. This ensures that blocking of non-specific antibody binding is maintained throughout.
Antibody Incubation
Primary antibody incubation
Unlike other antibody-based applications, such as immunohistochemistry, dilution of antibody for flow cytometry is typically based not on mass of antibody per volume of buffer, but on mass of antibody per number of cells in the sample. As with other applications, an optimal concentration must be empirically determined. Antibody may be diluted in flow cytometry assay buffer. Staining in small volumes improves access of antibody to cells in suspension. At the end of the incubation period, cells should be washed in staining or assay buffer at least three times to remove any unbound primary antibody.
Secondary antibody incubation
If an indirect detection technique is employed, primary incubation is followed by incubation with an appropriate dilution of secondary antibody specific for the isotype of each primary antibody used. In multicolor detection experiments, fluorophores of sufficiently different wavelengths or colors must be selected for each secondary antibody to permit the differentiation of signal from each target. Incubation with secondary antibody must be carried out in the dark to protect light-sensitive fluorophores and, as before, samples should be maintained on ice and centrifuged at 4°C.
Fluorochromes
Many antibodies used in flow cytometry are directly conjugated to a fluorochrome; however, many unlabeled primary antibodies are routinely used in combination with labeled secondary antibodies. The two most commonly used fluorochromes in flow cytometry are fluorescein isothiocyanate (FITC) and phycoerythrin (PE). The two key properties of these dyes that make them preferred tags are that they are both excited with a 488 nm laser and that their emission spectra are distinct, with FITC at 530 nm (green) and PE at 570 – 575 nm (orange). Advances in fluorochrome chemistry and flow cytometry instrumentation have made multiple simultaneous cell labeling and sorting possible beyond the original two dyes.

Figure 1.FluoroFinder® Spectra Viewer output of excitation and emission wavelengths of various fluorophores. From left to right, mFluor 450, Alexa Fluor 488, PE, APC, iFluor 700, and iFluor 750.
The maximum number of variables that can be examined in any given flow cytometry experiment centers on the number of light sources and detectors available. A flow cytometer with multiple lasers gives researchers the ability to simultaneously use fluorophores that do not share the same excitation spectrum, expanding the number of antibodies that can be employed. In addition, combining multiple lasers with multiple channels can even differentiate between two fluorophores with distinct excitation, but similar emission spectra as devices with parallel laser arrangements can collect distinct emission signals from a single particle as it is excited sequentially by each laser.
Tips for selecting appropriate fluorochromes:
- Choose the brightest set of fluorochromes for your instrument configuration.
- Depending on instrument configuration, choose fluorochromes to minimize the spectral overlap.
- Reserve the brightest fluorochromes for weaker antibodies and vice versa.
- Avoid spillover from bright cell populations into detectors requiring high sensitivity for those populations.
- Avoid tandem dye degradation and consider its impact on your results.
To learn more about selecting appropriate fluorochromes, see our guides on choosing the right fluorochrome for your flow cytometry experiment and building the optimal panel for multicolor flow cytometry analysis.
Fixation and storage
Once surface antigen staining has been completed, cells may be fixed in paraformaldehyde in phosphate-buffered saline instead of resuspending for acquisition. This is useful when samples cannot be acquired immediately after staining, as it allows cells to be stored overnight at 4°C. Afterward, the fixative should be diluted, and the cells washed at least twice. Although acquisition immediately following staining is recommended, fixed cells that have been stored at 4°C and protected from light may be acquired up to 48 hours after fixation.
Intracellular targets: cell permeabilization
When the protein(s) of interest is intracellular, fixation of cells after surface staining is necessary to increase structural strength so that the cells can withstand the subsequent permeabilization needed to allow antibodies access to intracellular antigen. Cells that have been fixed and washed may be permeabilized by incubation with a suitable detergent in phosphate-buffered saline for no more than 15 minutes at room temperature before diluting the detergent solution and washing once. Non-ionic detergents such as saponin are recommended. Permeabilization must be maintained during all steps involving antibodies or streptavidin, and this is achieved by including detergent in the staining buffer throughout subsequent staining up to and including the fluorophore incubation step. Detection of intracellular antigen by flow cytometry otherwise follows the principles and procedures outlined above and may be direct, indirect, or direct/indirect with signal enhancement.
Data Acquisition
Most current flow cytometers are accompanied by the software necessary to acquire and transform the signals generated by the particle characteristics as each cell passes by the detector. These software programs usually include components that aid in organizing the experiment, a feature that is of particular importance when analyzing a variety of samples from multiple specimens.
For multicolor experiments, it is critical to set parameters for compensation, in recognition of the wavelength overlap amongst spectra of various fluorophores used in the experiment. Compensation calibrates each fluorophore’s spectrum and allows for subtraction of signals from adjacent channels due to spectral overlap.
If your experiment is not yielding high-quality results, check out our Flow Cytometry Troubleshooting Guide for tips on solving common challenges.

Figure 2. The two-parameter flow cytometry plot shows how light-scattering characteristics of cells are used to identify cell populations of interest, here by their size (forward scatter) and intracellular complexity (side scatter). Gates, or regions defined and applied by the user, are then used to identify subpopulations of interest (here, the red oval identifies the likely viable population) for focused analysis.
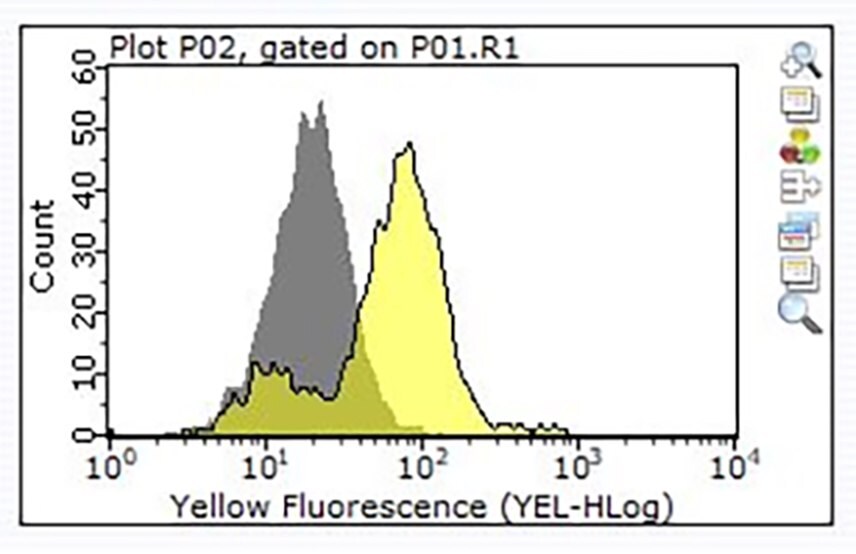
Figure 3. Staining of one million Raji cells performed using 1 μg of Cat. No. ZRB1564, Anti-CD24, clone 3N22 ZooMAb® Rabbit Monoclonal (Yellow histogram) or the equivalent amount of a Rabbit IgG isotype control (Grey histogram), followed by a PE-conjugated Donkey Anti-Rabbit IgG secondary antibody.
Para seguir leyendo, inicie sesión o cree una cuenta.
¿No tiene una cuenta?