P8129
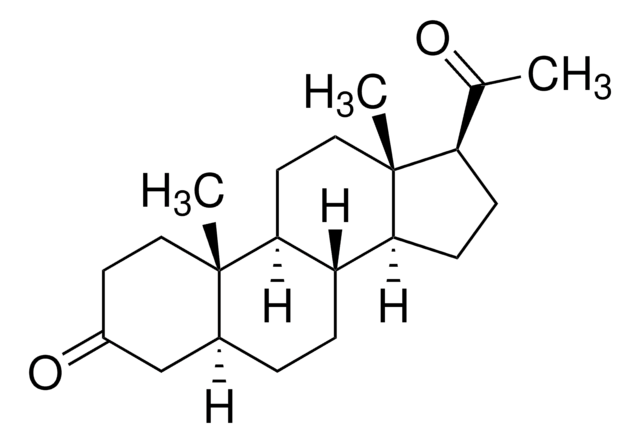
5β-Pregnan-3α-ol-20-one
Sinónimos:
3α-Hydroxy-5β-pregnan-20-one, 3α-Hydroxy-5β-tetrahydroprogesterone, Pregnanolone
About This Item
Productos recomendados
biological source
synthetic (organic)
Quality Level
assay
≥98% (TLC)
form
powder
solubility
chloroform: 50 mg/mL, clear, colorless
shipped in
ambient
storage temp.
room temp
SMILES string
[H]C12CCC3C4CCC(C(C)=O)C4(C)CCC3C1(C)CC[C@H](O)C2
InChI
1S/C21H34O2/c1-13(22)17-6-7-18-16-5-4-14-12-15(23)8-10-20(14,2)19(16)9-11-21(17,18)3/h14-19,23H,4-12H2,1-3H3
InChI key
AURFZBICLPNKBZ-UHFFFAOYSA-N
Gene Information
rat ... Gabra2(29706)
Biochem/physiol Actions
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico