39931
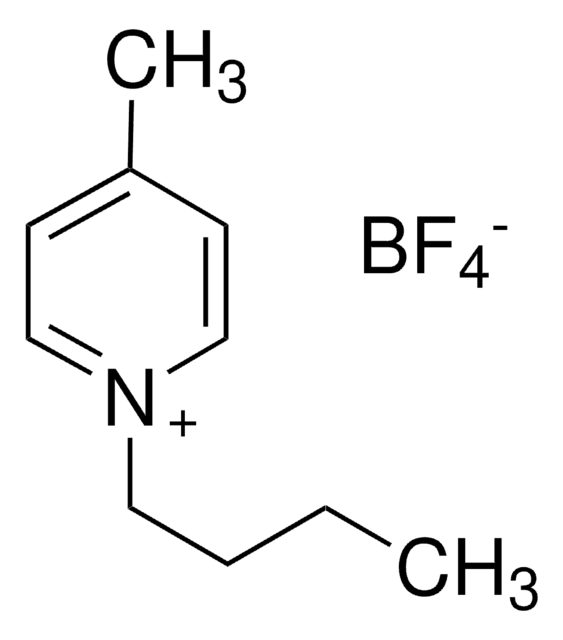
1-Butyl-3-methylimidazolium tetrafluoroborate
for catalysis, ≥98.5% (HPLC)
Sinónimos:
BMIMBF4
About This Item
grade
for catalysis
Quality Level
assay
≥98.5% (HPLC)
impurities
≤0.05% water
refractive index
n20/D 1.52
density
1.21 g/mL at 20 °C (lit.)
anion traces
bromide (Br-): ≤25 mg/kg
chloride (Cl-): ≤25 mg/kg
nitrate (NO3-): ≤25 mg/kg
phosphate (PO43-): ≤25 mg/kg
sulfate (SO42-): ≤10 mg/kg
SMILES string
F[B-](F)(F)F.CCCCn1cc[n+](C)c1
InChI
1S/C8H15N2.BF4/c1-3-4-5-10-7-6-9(2)8-10;2-1(3,4)5/h6-8H,3-5H2,1-2H3;/q+1;-1
InChI key
LSBXQLQATZTAPE-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
Application
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 3 Oral - Aquatic Chronic 2 - Eye Irrit. 2 - Skin Irrit. 2
Storage Class
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
wgk_germany
WGK 3
flash_point_f
550.4 °F
flash_point_c
288 °C
ppe
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Ionic Liquids have been thoroughly investigated as solvents in most types of catalytic reactions. Their merit lies in the ease with which their physical–chemical properties can be tuned by varying either the anion, the cation, or its substitution pattern.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico