An increased interest in aminophosphine type ligands used for asymmetric synthesis has been witnessed. This growth in popularity of aminophosphine ligands in asymmetric synthesis is in part due to the growing number of convenient synthetic pathways leading to useful ligand sets.1 Several groups have been using amino acids as precursors to synthesize these ligands. Researchers at Kanata have synthesized several sets of aminophosphine ligands showing great reactivity and selectivity in a wide array of enantioselective reactions.
Ruthenium Hydrogenation Catalysts
A growing area of application for aminophosphine ligands in asymmetric synthesis is in ruthenium-catalyzed hydrogenations.4 This process is integral in the preparation of alcohols and amines, which are essential in the pharmaceutical, agrochemical, material, and fine chemicals industries.
Chen et al. have described the use of ferrocenylaminophosphines in the ruthenium-catalyzed asymmetric hydrogenation of acetonaphthone.4 Using precatalysts [RuCl2(benzene)]2 and the ferrocenyl based aminophosphine ligand, they found that the hydrogenation proceeded quickly with reasonable enantioselectivity (Scheme 1).
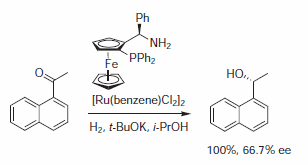
Scheme 1.
Abdur-Rashid et al. reported the synthesis of cis-2-tert-butylcyclohexyl alcohol using bis-2-(diphenylphosphino)ethylamine ruthenium dichloride as a catalyst.5 Excellent selectivities were reported yielding only the cis-product (Scheme 2).
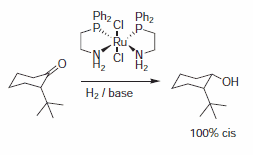
Scheme 2.
References
To continue reading please sign in or create an account.
Don't Have An Account?