R106
Ro 16-6491 hydrochloride
solid
Synonym(s):
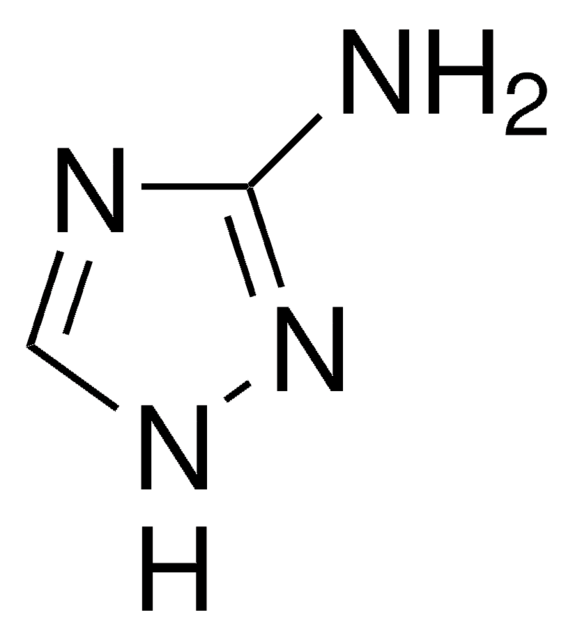
N-(2-Aminoethyl)-4-chlorobenzamide hydrochloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H11ClN2O · HCl
CAS Number:
Molecular Weight:
235.11
MDL number:
UNSPSC Code:
12352200
PubChem Substance ID:
Recommended Products
form
solid
color
white
mp
216-217 °C
solubility
H2O: soluble
SMILES string
Cl.NCCNC(=O)c1ccc(Cl)cc1
InChI
1S/C9H11ClN2O.ClH/c10-8-3-1-7(2-4-8)9(13)12-6-5-11;/h1-4H,5-6,11H2,(H,12,13);1H
InChI key
ARUMZUNJBHSOQQ-UHFFFAOYSA-N
Gene Information
human ... MAOB(4129)
Biochem/physiol Actions
Selective, reversible, orally-active MAO-B inhibitor which is devoid of indirect sympathomimetic activity.
comparable product
Product No.
Description
Pricing
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documents section.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Xi Jun He et al.
Neurotoxicology, 29(6), 1141-1146 (2008-07-09)
Neurotoxic effects of MPTP on the nigrostriatal dopaminergic system are thought to be initiated by 1-methyl-4-phenylpyridinium (MPP+), a metabolite formed by the monoamine oxidase (MAO)-B-mediated oxidation of MPTP. We previously reported that the administration of MPTP induced apoptosis in migrating
A M Cesura et al.
Journal of neural transmission. Supplementum, 32, 165-170 (1990-01-01)
The selective, reversible inhibitors of monoamine oxidase (MAO) moclobemide and Ro 41-1049 (selective for MAO-A), as well as of Ro 16-6491 and Ro 19-6327 (selective for MAO-B) inhibited the enzyme with an initial competitive phase, followed by a time-dependent inhibition
A M Cesura et al.
European journal of pharmacology, 162(3), 457-465 (1989-03-29)
This study demonstrated the existence of specific binding sites for [3H]Ro 19-6327 in human platelet membranes. This compound is a novel, time-dependent inhibitor of monoamine oxidase type B (MAO-B) and is structurally closely related to [3H]Ro 16-6491. The density of
G E Handelmann et al.
Pharmacology, biochemistry, and behavior, 34(4), 823-828 (1989-12-01)
The N-methyl-D-aspartate receptor complex appears to play an important role in processes of learning and memory. The presence of a glycine modulatory site at this complex has recently been established and suggests that glycinergic neurotransmission may influence these cognitive functions.
H H Keller et al.
Naunyn-Schmiedeberg's archives of pharmacology, 335(1), 12-20 (1987-01-01)
The inhibition of monoamine oxidase (MAO) in rat liver and brain by the short-acting MAO-A inhibitors moclobemide (Ro 11-1163 = p-chloro-N-[2-morpholinoethyl]benzamide) and brofaremine and by the short-acting MAO-B inhibitors Ro 16-6491 (N-[2-aminoethyl]-p-chloro-benzamide) and almoxatone, administered p.o. at roughly equieffective doses
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service