All Photos(3)
About This Item
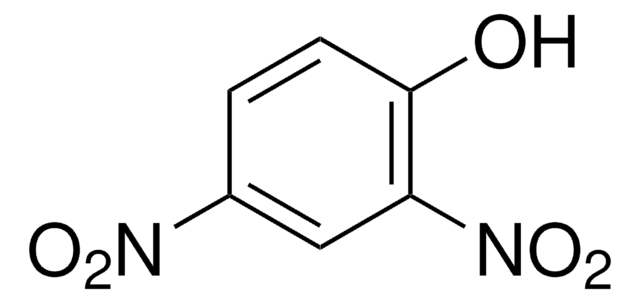
Linear Formula:
NO2NHC(=NH)NH2
CAS Number:
Molecular Weight:
104.07
Beilstein:
1756640
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
contains
20-25% water
Quality Level
mp
239 °C (dec.) (lit.)
SMILES string
NC(=N)N[N+]([O-])=O
InChI
1S/CH4N4O2/c2-1(3)4-5(6)7/h(H4,2,3,4)
InChI key
IDCPFAYURAQKDZ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Reactant involved in the synthesis of:
Reactant involved in:
- 1,5-Disubstituted-1,3,5-hexahydrotriazine-2-(N-nitro)imines with insecticidal activity
- Hexahydrotriazine-N-nitroimine analogs with insecticidal activity via Mannich reactions
Reactant involved in:
- The study of the effects of vessel materials on exothermic decomposition energy
- Nitration of arenes
- Nitration of (nitrimino)tetrahyrdooxadiazine and (nitrimino)hexahydrotriazine
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Desen. Expl. 4
Storage Class Code
4.1B - Flammable solid hazardous materials
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Toxicity and uptake of nitroguanidine in plants.
J J Heitholt et al.
Bulletin of environmental contamination and toxicology, 44(5), 751-758 (1990-05-01)
Rajib Karmakar et al.
Journal of agricultural and food chemistry, 57(14), 6369-6374 (2009-06-19)
The metabolism of thiamethoxam [(EZ)-3-(2-chloro-1,3-thiazol-5-yl-methyl)-5-methyl-1,3,5-oxadiazinan-4-ylidene (nitro) amine] was investigated in whole plant, callus, and heterotrophic cell suspension culture of aseptically and field grown tomato (Lycopersicon esculentum Mill.) plants. The structure of the metabolites was elucidated by chromatographic (HPLC) and spectroscopic
[Research and determination of occupational exposure limits of nitroguanidine in air of working places].
Wen-xia Du et al.
Zhonghua lao dong wei sheng zhi ye bing za zhi = Zhonghua laodong weisheng zhiyebing zazhi = Chinese journal of industrial hygiene and occupational diseases, 28(5), 380-382 (2010-09-22)
Ryan A Dick et al.
Chemical research in toxicology, 19(1), 38-43 (2006-01-18)
The nitroguanidine or nitromethylene moiety of the newest major class of insecticides, the neonicotinoids, is important for potency at insect nicotinic receptors and selectivity relative to mammalian receptors. Aldehyde oxidase (AOX) was recently identified as the imidacloprid nitroreductase of mammalian
Nancy N Perreault et al.
Environmental science & technology, 46(11), 6035-6040 (2012-05-09)
Nitroguanidine (NQ) is an energetic material that is used as a key ingredient of triple-base propellants and is currently being considered as a TNT replacement in explosive formulations. NQ was efficiently degraded in aerobic microcosms when a carbon source was
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service