Multiplex Quantification of Infliximab and Adalimumab in Human Serum by LC-MS/MS Using Full-Length Stable Isotope Labeled Internal Standards
Pegah R. Jalili & Kevin Ray
MilliporeSigma, St. Louis, MO
Abstract
Infliximab and Adalimumab monoclonal antibodies (mAbs) are used widely to treat rheumatoid arthritis, psoriatic arthritis and many autoimmune diseases by binding to tumor necrosis factor-alpha (TNFα) to reduce the inflammatory response. Clinical responses are different among patients due to inadequate amount of drug in blood and the formation of autoantibodies, which can also interfere with ELISA assays. Therefore, there is a growing demand for reliable LC-MS/MS assays to support quantification of serum Infliximab and Adalimumab mAbs. The accurate quantitation of these antibodies is enabled by early introduction of internal standards that behave identically to the native target proteins throughout the analytical workflow. We have developed full-length stable isotope labeled (SIL) Infliximab and Adalimumab mAb internal standards, which allow significant improvements in accuracy and reproducibility in routine quantification of serum Infliximab and Adalimumab using LC-MRM assay.* We demonstrate a lower limit of quantitation, without immunoenrichment, of 500 ng/mL for Infliximab and Adalimumab with less than 15% CV and ± 15% accuracy.
Methods
SIL-Infliximab and SIL-Adalimumab were expressed in CHO cells which were grown in serum-free media enriched with 13C615N4 Arginine and 13C615N2 Lysine. The SIL labeled mAbs were analyzed at the intact protein level and after trypsin digestion. Intact mass analysis (SEC-MS) was used to confirm the amino acid composition of the protein and level of glycosylation. The sequence and isotope incorporation were determined at the peptide level after trypsin digestion. For quantification, samples were prepared by spiking 20 µg/mL of SIL-Infliximab and SIL-Adalimumab as internal standards into human serum containing 0.5 - 100 µg/mL of target antibodies. Samples were precipitated by adding saturated ammonium sulfate, reconstituted with 50 mM ammonium bicarbonate, and digested. Tryptic peptides were separated on a Supelco BIOshell A160 Peptide C18, 2.7 µm fused core particle column; 10 cm x 500 µm. Detection was performed in MRM mode on Sciex QTRAP 5500 system. Transitions of four unique Infliximab peptides and one unique Adalimumab peptide were monitored.
SEC-MS Analysis
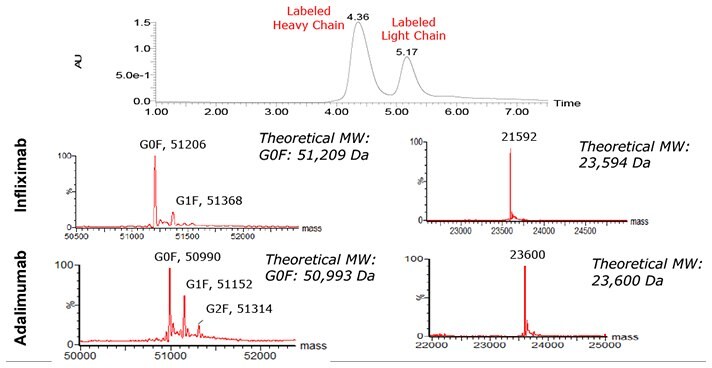
Figure 1.UV trace and deconvoluted mass spectra resulting from SEC-MS analysis of the reduced Infliximab and Adalimumab.
Isotopic Incorporation Analysis

Figure 2.Incorporation of 13C615N4 labeled Arginine and 13C615N2 Lysine for selected peptides of Infliximab and Adalimumab. Incorporation was demonstrated to be greater than 99%.
LC-MRM Analysis of Infliximab and Adalimumab
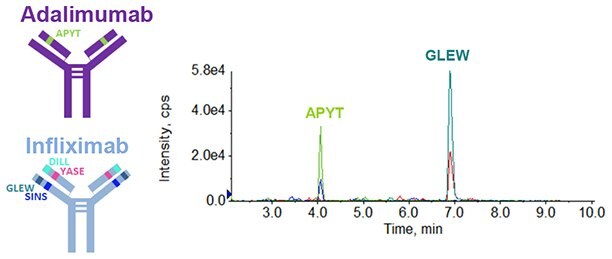
Figure 3.Extracted ion chromatogram (XIC) of one unique Infliximab peptide and one unique Adalimumab peptide.
Quantification
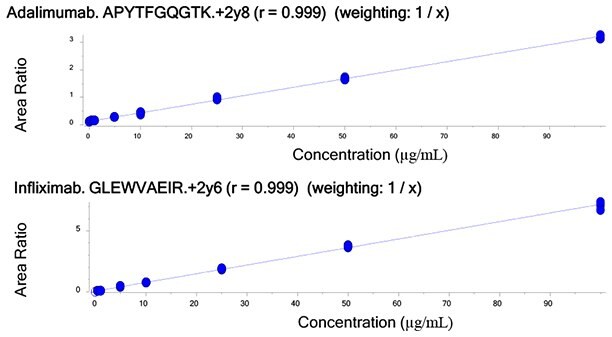
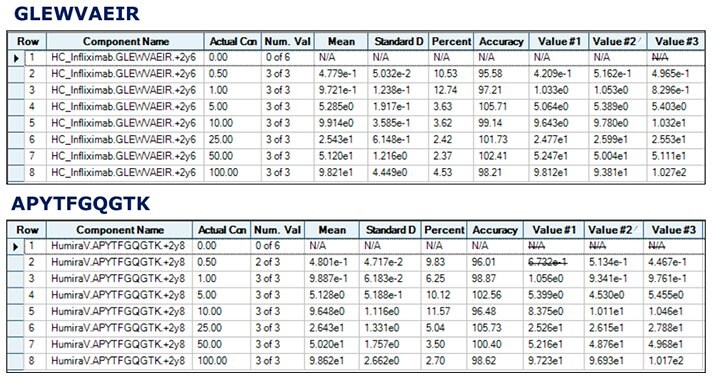
Figure 4.Calibration curves of unique tryptic peptides of Infliximab and Adalimumab obtained by spiking 20 µg/mL of IS into human serum containing 0.5 - 100 µg/mL target antibodies.
Summary
- Stable isotope labeled full-length Infliximab and Adalimumab mAbs have been produced with high purity and isotopic incorporation > 99%.
- We demonstrate that the use of full length SIL-Infliximab and SIL-Adalimumab internal standards allows sensitive, accurate, and reproducible multiplex quantification of target antibodies in human serum.*
References
계속 읽으시려면 로그인하거나 계정을 생성하세요.
계정이 없으십니까?