Enzymes for Antibody Characterization
Robert Gates
Antibodies now make up the fastest growing category of therapeutic drugs. Even with today's highly-defined recombinant humanized antibodies, the control and analysis of micro-heterogeneities relating to functional impact have become critical to the quality by design paradigm.1 Alain Beck and his colleagues at the Centre d'Immunologe at Pierre Fabre are leading the efforts to identify and characterize the criteria for therapeutic antibody analysis and quality control.2,3
Among the areas of antibody peptide and glycan analysis currently used or under investigation:
- Glycoform variability mainly relating to heterogeneous N-linked glycan populations
- Charge variants resulting from several types of protein modifications
- Amino acid modifications such as Asparagine deamidation, Aspartate isomerization, and formation of N-terminal pyroglutamate
- Cysteine-related variants and oxidation states
- Positive or basic charge patches
- Molecular mass and primary amino acid variants including C-terminal lysine clipping
- Secondary through quaternary protein structure and antigen complex variations
- Dimerization and aggregation
- Contamination with host cell proteins
Several modalities of Mass Spectroscopy (MS) analysis have emerged as core technologies for antibody peptide and glycan characterization. The following are some examples:
- ESI, MALDI-TOF, Native, and Hydrogen/Deuterium Exchange MS methods for intact antibody and antibody/antigen complex analysis.
- LC-MS and LC-MS/MS utilizing stable isotope labeled peptide internal standards for antibody quantification.
- Middle-up and middle-down LC-MS analysis of chemically or enzymatically generated antibody fragments.
Dr. Beck and others are investigating not only new methodologies for improving the quality control of existing therapeutic antibodies, but for the development of biosimilars, biobetters, and next generation antibodies.2 In his recent review article, Dr. Beck highlights two new enzymes from Genovis Enzyme Technologies for antibody-specific glycan and peptide analysis:3
- FabRICATOR® IdeS Protease
- IgGZERO™ (EndoS Glycanase)
FabRICATOR IdeS Protease for Improved Fc Fragment Generation
Peptide hydrolysis of antibodies has historically utilized proteases such as papain, pepsin, and endoproteinase Lys-C, which can suffer from limited specificity. Recently, the immunoglobulin-degrading IdeS cysteine protease (FaBRICATOR) from Streptococcus pyogenes has provided an improvement in specificity and digestion time. Since FabRICATOR cleaves under the hinge domain, antibodies with glycosylated Fab regions will yield glycans associated with two separate peptide fragments. This enables more effective glycan analysis, particularly relating to the effector functions of Fc fucosylation and galactosylation.4
Because of its exceptionally high specificity for IgG protein sequences, Goetze, A.M. et al. were able to utilize the FaBRICATOR enzyme to cleave IgG in serum to produce Fc fragments for analysis by LC-MS to identify individual haplotypes.5
Advantages of using FabRICATOR for F(ab')2 or Fab' production
- Faster digestion times – IgG cleaved within 30 minutes compared to digestion times as long as 12 hours for pepsin and papain.
- Optimized formulation and protocol specific for IgG fragmentation
- High specificity compared to other proteases yielding the same fragment under variable conditions.
- Digestion performed under mild conditions at physiological pH with no negative effect on immunoreactivity of the generated fragments.
- Antibodies Compatible with FabRICATOR enzyme: Human IgG, Humanized IgG, Chimeric IgG, Monkey, Rabbit, Sheep, Mouse IgG2a, and Mouse IgG3

Figure 1.FabRICATOR cleaves IgG isotypes just below the hinge region, generating an intact F(ab')2 fragment and a Fc fragment.

Figure 2.Cleavage of a therapeutic antibody with FabRICATOR just below the hinge region, generating an intact F(ab')2 fragment and a Fc fragment. This provides simplified MS analysis of antibodies that have two different glycosylation sites on the heavy chain, by yielding two separate peptide fragments, each with one glycosylation site.
IgGZERO (EndoS Glycanase) for Improved N-Linked Glycan Hydrolysis
Enzymatic glycan hydrolysis of antibodies has historically utilized glycanases such as PNGase F, and other endo and exoglycosidases. IgGZero (EndoS), also from Streptococcus pyogenes, has demonstrated exceptional hydrolytic activity specific for IgG bound glycans.6,7 The enzyme cleaves the chitobiose core of the glycan on IgG from various sources, such as human, rabbit, mouse, Rhesus monkey, goat, sheep, rat, horse, dog, porcine, and more.
Advantages of IgGZERO
- IgGZERO is the first known bacterial enzyme with a unique specificity for native IgG, no denaturation of the substrate is required. The activities of other endoglycosidases, such as PNGase F, require or are enhanced by denaturation of the glycoprotein substrate.
- Removal of IgGZERO is extremly easy as the recombinant enzyme contains an N-terminal histidine tag.
- IgGZERO efficiently cleaves bound IgG from IgG Fc receptors without producing additional damage to native cells.
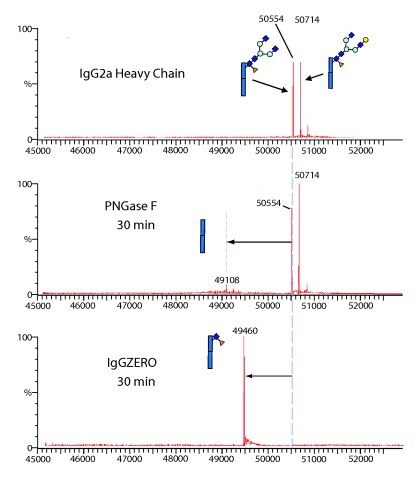
Figure 3.IgGZERO shows specific endoglycosidase activity on native IgG because it hydrolyzes the conserved glycans attached to Asp297 on IgG heavy chains.
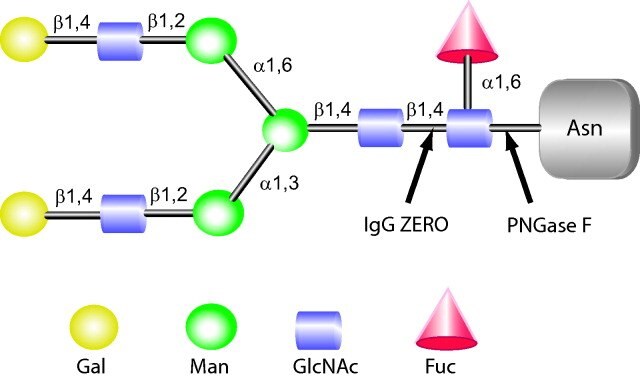
Figure 4.Cleavage sites of IgGZERO and PNGase F
Summary
Therapeutic antibody analysis is evolving, particularly as liquid chromatography, electrophoresis, and MS workflows are being refined. FabRICATOR and IgGZero enzymes are adding another dimension of accuracy and ease-of use to these methods.
Find out more about products for antibody characterization including the Genovis enzymes, and Maxi and Minispin Columns on our Antibody Characterization Website.
References
계속 읽으시려면 로그인하거나 계정을 생성하세요.
계정이 없으십니까?