Solubility enhancement of hydrophobic drugs via drug-loaded micelles using biodegradable PEG- polyester diblock copolymers
One of the common difficulties with intravenous drug delivery is low solubility of the drug. The requirement for large quantities of saline to dissolve such materials limits their clinical use, and one solution for this problem that has recently generated interest is the formation of drug-loaded micelles. (In this article “micelle” is used to indicate a spherical structure with a diameter of <1 µm, often referred to as a nanoparticle or liposome, which can be loaded with a drug.) This is accomplished because the drug has a high solubility in the micelle, and the micelle has a high solubility in the solvent. Amphiphilic diblock copolymers consisting of a hydrophilic bock, such as poly(ethylene glycol) (PEG), and a hydrophobic block, such as an alkyl chain, polylactide, or polyglycolide have the capacity to form micelles. The micelle shown in Figure 1 illustrates how a hydrophobic drug is surrounded by the hydrophobic blocks of the polymers inside a micelle, while the hydrophilic PEG blocks are exposed to hydrophilic solvent. The phase segregation of polymer-blocks into a micellar morphology creates an environment where hydrophobic molecules can dissolve in the hydrophobic core, despite the limited solubility in the solvent (which is usually water or saline for biological applications). In research that will lead to in vivo testing, the selection of biocompatible polymers is crucial. PEGs are commonly employed as biocompatible hydrophilic polymers, and polylactides (PLA) and polyglycolides (PGA) degrade over time into acids that can be safely eliminated from a living system .1-4
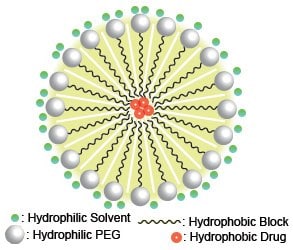
Figure 1. Schematic example of drug-loaded micelles
Coumarin-6 (C6) is traditionally used as a laser dye due to its highly fluorescent nature. Recently, however, it is commonly used as a model hydrophobic drug for studies involving controlled release and tracking of localized delivery. The native solubility of C6 in water is 0.25 µg/mL, which makes it a good model for hydrophobic drugs such as paclitaxel, everolimus, and others. Also, since this material is bright yellow it is easy to observe delivery with the naked eye.
Solubility test of hydrophobic drug model (Coumarin 6) in water in conjunction with polyethylene glycol-polylactide (PEG-PLA) diblock copolymer
Poly(ethylene glycol) methyl ether-block-poly(D,L-lactide) decane (mPEG-PDLLA-decyl) [Product No. 764736] is a diblock copolymer of hydrophilic PEG and hydrophobic poly(D,L-lactide) (PDLLA) with a 10 carbon alky end-cap. The nominal block weight of the PEG is 2,000 Da and PDLLA is 2,000 Da. The purpose of the alkyl end-cap is to improve the hydrophobicity and solubilizing power of the micelle interior (i.e. a higher drug loading capability). The polymer is directly soluble in water up to 10 % w/v. Dissolution of this polymer into water improves the capacity of the solution to dissolve drugs directly.
![Chemical structure of Methoxypoly(ethylene glycol)-block-poly(DL-lactide)decane (mPEG-PDLLA-decyl) [Product No. 764736] Methoxypoly(ethylene glycol)-block-poly(DL-lactide)decane (mPEG-PDLLA-decyl)](/deepweb/assets/sigmaaldrich/marketing/global/images/technical-documents/articles/materials-science-and-engineering/drug-delivery/peg-pla/peg-pla.jpg)
Figure 2. Chemical structure of Methoxypoly(ethylene glycol)-block-poly(DL-lactide)decane (mPEG-PDLLA-decyl) [Product No. 764736]
Test Method
Excess coumarin 6 (C6) solid was placed in a series of 2 mL microcentrifuge tubes. To each tube, ~1.5 mL of test solution was added and the tubes were incubated upright at 37 °C with 120 RPM orbital agitation overnight to equilibrate. The next day, 1 mL of each solution was first pulled into a pre-warmed syringe, and then pushed through a 0.45 µm PVDF filter to remove any particulates. The filtered solution was then diluted with 1 mL of ethanol and tested for absorbance at 460 nm on a Genesys20 spectrophotometer. The absorbance was compared with a set of known C6 standards made by diluting C6 in pure ethanol and multiplied by dilution to obtain the concentration of C6 in each solution.
This method was followed for both freshly dissolved mPEG-PDLLA-decyl as well as a 1 % w/v mPEG-PDLLA-decyl solution that was incubated in water at 37 °C for 1 week prior to use. All tests were performed in triplicate. Confidence values were calculated based on t-test at 95% confidence level.
Results
The following results in Figure 3 show the relationship between mPEG-PDLLA-decyl w/v % in water and the resultant C6 solubility immediately after overnight agitation.
![This dilution series shows the increase in Coumarin-6 (C6) solubility (in µg/mL) with an increase in concentration of Methoxypoly(ethylene glycol)-block-poly(DL-lactide)decane (mPEG-PDLLA-decyl) [Product No. 764736] in water. Error bars are based on a t-test with a 95% confidence. Methoxypoly(ethylene glycol)-block-poly(DL-lactide)decane (mPEG-PDLLA-decyl)](/deepweb/assets/sigmaaldrich/marketing/global/images/technical-documents/articles/materials-science-and-engineering/drug-delivery/dilution-series/dilution-series.jpg)
Figure 3. This dilution series shows the increase in Coumarin-6 (C6) solubility (in µg/mL) with an increase in concentration of Methoxypoly(ethylene glycol)-block-poly(DL-lactide)decane (mPEG-PDLLA-decyl) [Product No. 764736] in water. Error bars are based on a t-test with a 95% confidence.
As can be seen, the impact of the polymer concentration appears to generate a linear increase in C6 solubility over the range from XX% to 2.5% polymer. The solubility of C6 increases from less than 1 µg/mL in water to more than 10 µg/mL with 2.5 % w/v mPEG-PDLLA-decyl, indicating a 40-50 fold increase in C6 solubility over a pure aqueous system. Next, the solubilizing power of the micelles over time was tested. The C6 solubility in mPEG-PDLLA-decyl immediately after overnight agitation was 5.42 ± 0.74 µg/mL, and after one week of incubation at 37 °C the solubility of C6 was 4.85 ± 0.46 µg/mL. The difference is statistically insignificant indicating minimal loss of dissolving power over the course of 1 week at 37 °C.
Use of mPEG-PLGA diblock copolymers to form drug-loaded micelles
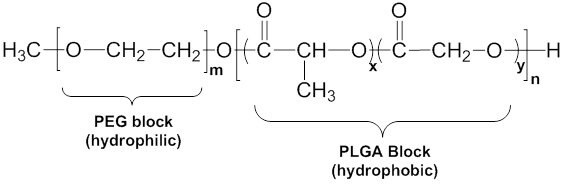
Figure 4. Generalized structure of methoxypoly(ethylene glycol)-block-poly(lactide-co-glycolide) (mPEG-PLGA).
Methoxypoly(ethylene glycol)-block-poly(lactide-co-glycolide) mPEG-PLGA (Figure 4) is a block copolymer which contains a methoxy end-capped hydrophilic PEG block and a hydrophobic block composed of poly(lactide-co-glycolide) (PLGA) with a hydroxyl end group. We offer a range of polymers with different molecular weights and different block ratios. Differences in molecular weight, block length, and ratio affect solubility and micelle properties. At high PLGA/PEG block weight ratios (i.e. in copolymers which contain a high fraction of hydrophobic PLGA), the polymer does not dissolve in water. It is only soluble in organic solvents such as dichloromethane (DCM). As the ratio of PLGA to PEG is decreased, the solubility of the copolymer increases, and the solubility properties are effectively tuned to result in specific sized micelles with specific solubilities.

Figure 5. Stirring paddle setup.
Method
Polymer-Drug Solution
The polymer mPEG-PLGA was dissolved in DCM at a concentration of 5% w/v. Separately 2.5 % w/v C6 was dissolved in DCM and added as 0.1 mL of C6 solution into 1 mL of polymer solution. This renders a solution such that the final ratio of drug/polymer was 5% w/w drug to polymer. As controls a negative control of pure DCM (no polymer) mixed with C6 was used.
Liposomes
A 22 mL scintillation vial was filled with 20 mL of distilled water. It was agitated using an overhead stirrer equipped with a screw-drive at a rate of 2000 RPM (Figure 5). While being stirred, 1.1 mL of each polymer-drug solution was added to the stirring water 100 µL at a time and the mixture/solution was left to stir for at least 1 hour to allow for DCM evaporation.
Screening
To remove any macroscopic scum layer, each solution was first passed through a folded paper filter (qualitative, fast) by gravity. Following this, each solution was loaded into a syringe and pushed through a 0.45 µm (450 nm) PVDF filter to eliminate any microparticulate manner. The filtered solution was considered to be the liposomal solution as anything larger than 450 nm had been excluded (Figure 6).

Figure 6.Screening to size.
After screening, solution stability was briefly tested by centrifuging at 2000 RPM for 1 min and then, allowing the solution to sit still overnight.
Results
Immediately after filtration, the solutions with and without mPEG-PLGA copolymer were visually inspected (pictured below in Figure 7). As you can see in the photo, the solution on the left with the solubilizing copolymer contained a visible concentration of yellow C6, and the negative control solution on the right, which did not contain any solubilizing copolymer, did not contain a visible concentration of C6. Almost all of the C6 had been filtered out of the negative control. In the absence of mPEG-PLGA, C6 lacks the solubility to be retained in water; however, when co-dissolved with a micelle forming polymer, it had high solubility in the micelle, and the micelle had solubility in water.

Figure 7. C6 solutions with (left) and without (right) stabilizing polymer.
These were diluted 1:10 in ethanol and tested by UV/Vis spectroscopy for C6 content at 469 nm and compared to known standards to obtain C6 content. The results are shown in Table 3 below. Please note that this does not necessarily represent the maximum possible loading of these polymers, simply the concentration achieved by following the protocol above. Stability was tested by centrifuging the material at 2,000 RPM for 1 min. No settling was observed indicating that the C6 was evenly dispersed in the aqueous phase and stabilized against settling.
Conclusions
Diblock copolymers such as Poly(ethylene glycol) methyl ether-block-poly(D,L-lactide) decane (mPEG-PDLLA-decyl) [Product No. 764736] and methoxypoly(ethylene glycol)-block-poly(lactide-co-glycolide) (mPEG-PLGA) can form micelles to enhance the solubility of hydrophobic molecules such as Coumarin-6 (C6). An increase in copolymer loading was shown to increase the C6 solubility, and this was shown to remain statistically constant over a period of at least a week. As the ratio of PLGA to PEG is changed, the solubility of the copolymer changes, and the solubility properties are effectively tuned to result in specific sized micelles with specific solubilities. The controllable properties of these copolymers are crucial to developing new techniques to deliver hydrophobic drugs.
Precautions and Disclaimers
These products are for R&D use only, not for drug, household, or other uses. Please consult the Safety Data Sheet for information regarding hazards and safe handling practices.
Storage and Stability
Polymers are biodegradable upon contact with moisture. Store at -20 °C in the presence of desiccant.
References
계속 읽으시려면 로그인하거나 계정을 생성하세요.
계정이 없으십니까?