Asymmetric Reduction of Ketones
William Sommer
ChemFiles 2008, 8.6, 5.
The enantioselective reduction of ketones to alcohols gives access to a pool of chiral building blocks that can be used for the synthesis of natural products. Adolfsson and co-workers reported a novel class of ligands, based on a pseudo-dipeptide,1 for the mild and efficient reduction of ketones using 2-propanol as a hydrogen source. The ligand is used with [{RuCl2(p-cymene)}2] in presence of NaOH for the asymmetric reduction of a variety of acetophenone derivatives. Excellent yields and ee’s were reported (Scheme 1).
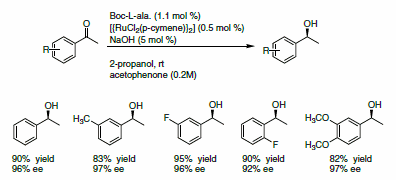
Scheme 1.
Materials
Loading
References
1.
Bøgevig A, Pastor IM, Adolfsson H. 2004. Highly Enantioselective Ruthenium-Catalyzed Reduction of Ketones Employing Readily Available Peptide Ligands. Chem. Eur. J.. 10(1):294-302. https://doi.org/10.1002/chem.200305553
계속하려면 로그인하세요.
계속 읽으시려면 로그인하거나 계정을 생성하세요.
계정이 없으십니까?