Purer Fmocs Means Purer Peptides
New stunning specifications for the 20 standard Fmocs
All peptide chemists recognize the key to obtaining high purity peptides is to maximize efficiency of every synthetic step. However, not everyone realizes that obtaining high purity peptides also depends on using starting materials of the highest quality - small improvements in starting material quality can result in dramatic improvements in peptide yield (Figure 1 shows effect of amino acid purity on step-wise yield). That is why we are pleased to introduce enhanced specifications for the standard 20 Fmoc-amino acid building blocks:
- HPLC Purity NOW ≥ 99%* [*Except Fmoc-Trp(Boc)-OH], with all significant amino acid related impurities quantified
- No impurites hidden under product peak gives confidence in product purity - Enantiomeric purity NOW ≥ 99.8%
- Free amine content ≤ 0.2 %
- Improved Fmoc stability during storage - Acetate content ≤ 0.02 %
- Negligible capping by-products
Purer Fmocs mean:
- Higher yields and more easily purified peptides
- More reproducible impurity profile of crude peptides so more easily optimized scale-up and less regulatory issues during GMP peptide manufacture
- Less synthesis failures and more easy trouble-shooting as you can have confidence in the integrity of the amino acid building blocks
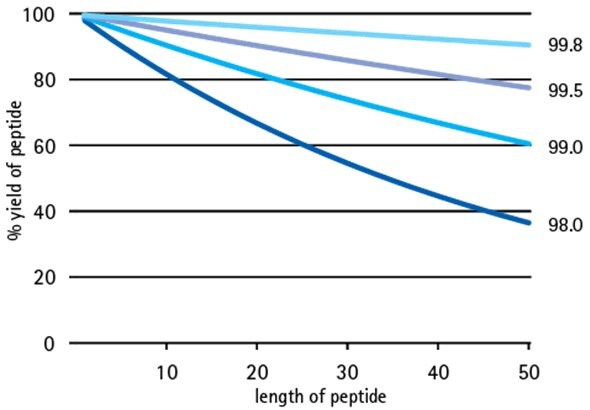
Figure 1.Effect of building block purity on step-wise peptide yield.
Is pure really pure? Can you be sure 99% HPLC purity really means your Fmoc is 99% pure?
What about the hidden impurities?
Synthesis of an Fmoc-amino acid produces predictable amino-acid related impurities, the presence of which can negatively effect the outcome of peptide synthesis.
How can you be sure these impurities are not hiding under your “99%” pure HPLC peak?
By performing HPLC analysis the Novabiochem® way: by employing characterized standards of all amino-acid related impurities and using highly optimized HPLC methods to ensure these are clearly separated from the Fmoc-amino acid, enabling their accurate quantitation.
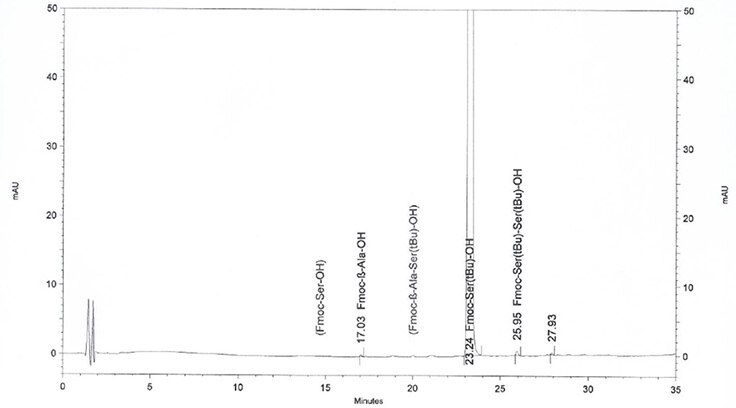
Figure 2.Actual HPLC profile of Fmoc-Ser(tBu)-OH, showing amino acid impurities clearly identified
But purity does not end with HPLC purity!
Standard HPLC columns cannot separate between D- and L-amino acids, so HPLC analysis of your peptide will always miss enantiomeric impurities. However, small amounts ofenantiomeric impurities in your Fmoc-amino acids will have a significant impact on the quality of the target peptide.
Other hidden impurities
Acetic acid: Fmoc-amino acids can contain traces of acetic acid, which can cause chain termination during synthesis. For this reason, Novabiochem specifies acetate content of less than 0.02% for all our 20 proteinogenic Fmoc-amino acids.
Free-amino acid content: Unlike other suppliers who use an inaccurate TLC-based assay, Novabiochem employs a quantitative GC-based method, to ensure a free amino acid content of less than 0.2%.
Introducing our new specifications
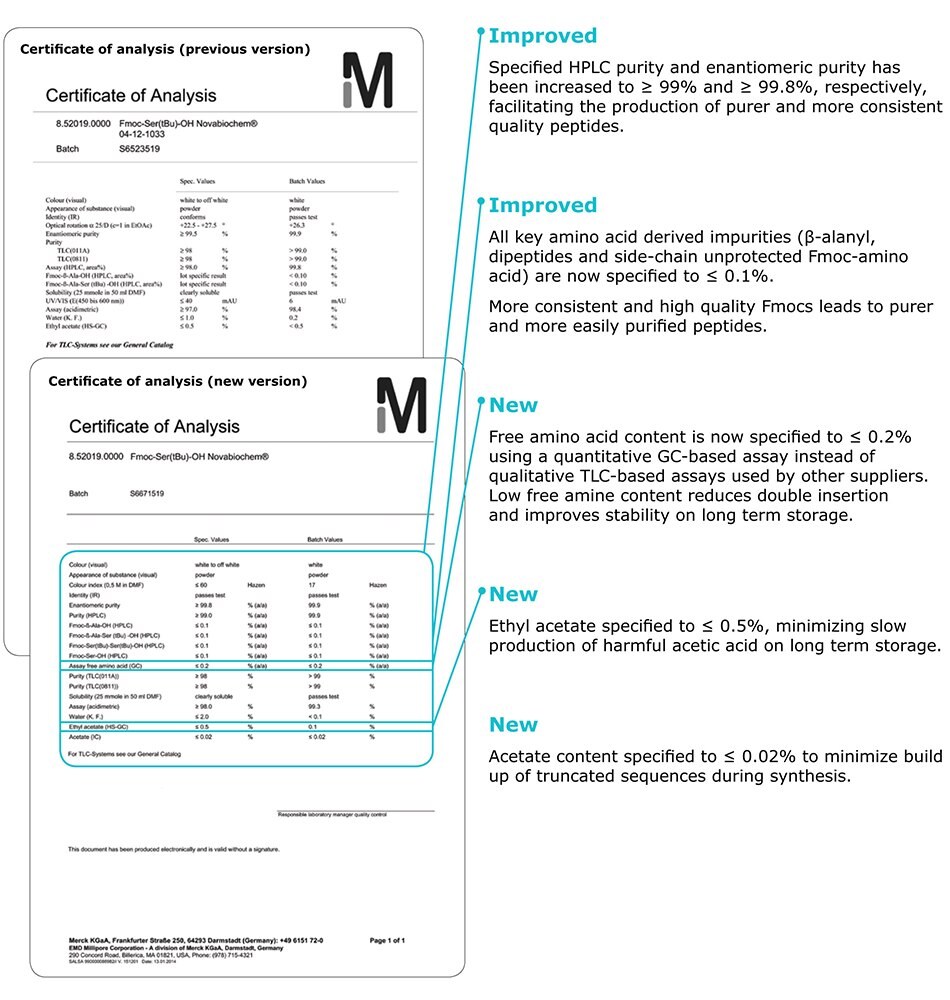
Specified HPLC purity and enantiomeric purity has been increased to ≥ 99% and ≥ 99.8%, respectively, facilitating the production of purer and more consistent quality peptides.
All key amino acid derived impurities (b-alanyl, dipeptides and side-chain unprotected Fmoc-amino acid) are
now specified to ≤ 0.1%.
More consistent and high quality Fmocs leads to purer and more easy to purify peptides.
Free amino acid content is now specified to ≤ 0.2% using a quantitative GC-based assay instead of qualitative TLC-based assay used by other suppliers. Low free amine content reduces double insertion and improves stability on long term storage.
Ethyl acetate specified to ≤ 0.5%, minimizing slow production of harmful acetic acid on long term storage.
Acetate content specified to ≤ 0.02% to minimize build up of truncated sequences during synthesis.
So where do these impurities come from?
The impurities present in Fmoc-amino acids are predictable and mainly arise from side reactions during the attachment of the Fmoc group to the amino acid.
These impurities arise from reaction of reagent used to introduce the Fmoc group with the already formed Fmoc-amino acid. The presence of these impurities leads double insertion of the target amino acid.
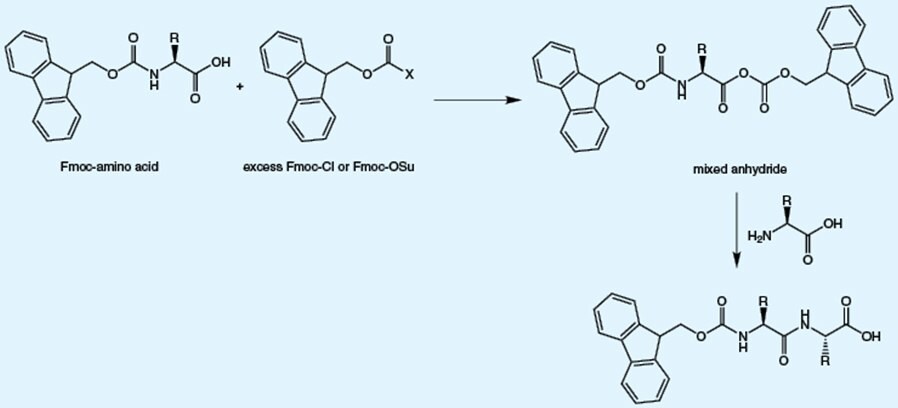
Ring opening and rearrangement of Fmoc-OSu, which is used for Fmoc introduction, results in the formation of β-alanyl impurities. The presence of these impurities leads to insertion of additional β-alanine residue (B) or β-alanine (A) instead of the target amino acid into the peptide.
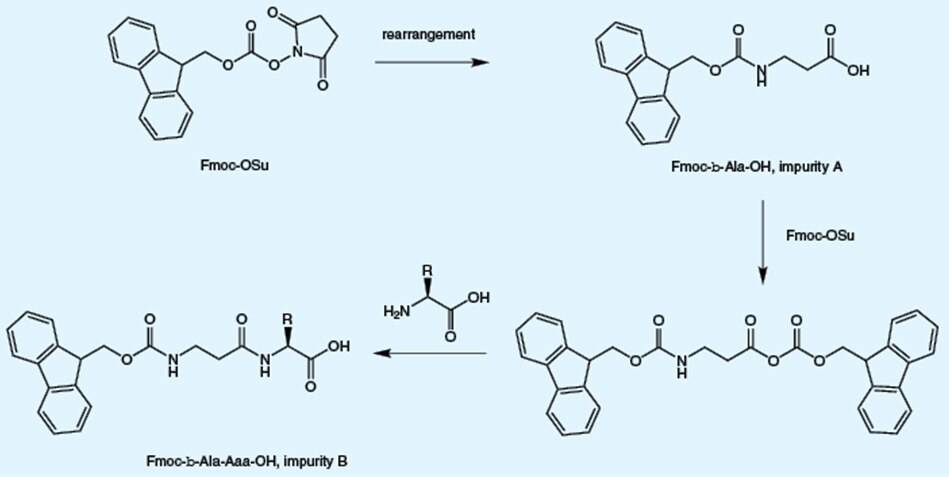
Free amino acids arise from incomplete reaction of the amino acid with the Fmoc reagent. The presence of free amino acid will lead to insertion of multiple copies of the target amino acid into the peptide chain and promote autocatalytic cleavage of the Fmoc group during storage of the Fmoc amino acid derivative. Unlike other suppliers which use an inaccurate TLC-based assay, Novabiochem® employs a quantitative GC-based method, to determine the levels of these impurities.
Acetic Acid: The hidden killer
Acetic contamination of Fmoc-amino acids results in truncation of the growing peptide during solid phase synthesis. Even traces can have a dramatic effect owing to the small molecule mass of acetic acid and its high reactivity.
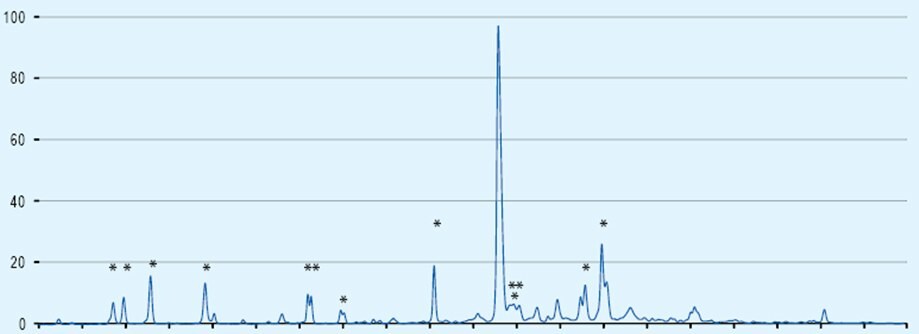
Consider Fmoc-Arg(Pbf)-OH contaminated with as little as 0.1% acetic acid. This translates to 1 mol% acetic acid contamination which does not sound too bad. However, when one considers that generally a 5-fold excess of reagents is used and the highly reactive acetic acid will always couple before the amino acid, then this can result in up to 5% chain termination each cycle. If you peptide contains 3 arginine residues than is 15% of chains will be terminated.
The presence of acetic acid in Fmoc-amino acids is very hard to detect. It is invisible to HPLC and can not be detected in trace quantities by 1H NMR. This is why we developed its methods for quantitation of acetic acid.
The effect of just 0.1% acetic contamination on the synthesis of a 21mer is illustrated in the HPLC chart below the truncated by-products are marked by an asterix
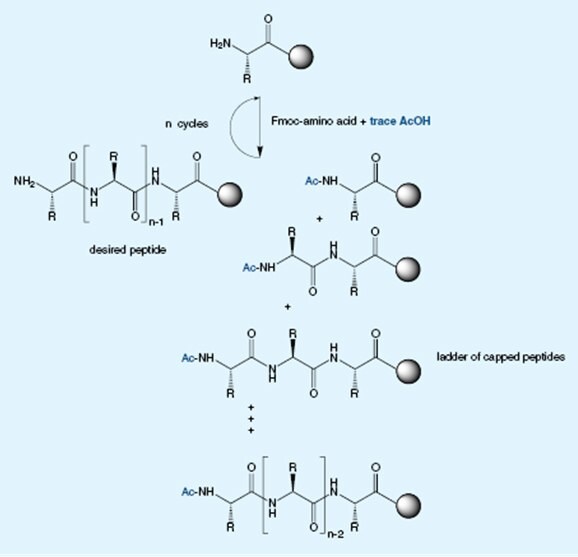
So where does the acetic acid come from?
Ethyl acetate is a common solvent used in the preparation and crystallization of Fmoc-amino acids. Hydrolysis of this solvent results in formation of acetic acid. Moreover, residual ethyl acetate left after crystallization can slowly transesterify with the solid Fmoc-amino acid, resulting in formation of acetic acid on storage. This is why we specify both acetate and ethyl acetate content of its 20 standard Fmoc-amino acids.

계속 읽으시려면 로그인하거나 계정을 생성하세요.
계정이 없으십니까?