C2-symmetric Chiral Bisoxazolines (BOX) Ligands
C2-symmetric chiral bisoxazolines (BOX) are privileged structures because they promote a great number of transformations with unprecedented selectivity.1 In 1991, in the same Journal of American Chemistry issue, two communications appeared by Evans2 and Corey.3 These two publications paved the way for a rapidly and ever-growing number of examples where BOX ligands are used successfully as chiral ligands.
Evans described the cyclopropanation of alkenes using diazo esters catalyzed by a complex formed of 2,2′-Isopropylidenebis[(4S)-4-tert-butyl-2-oxazoline] (Product 406147) and Cu(I)OTf. With the ester derived from 2,6‑di-tert-butyl-4‑methylphenol (BHT) it was found that the increased steric demand displays increased trans selectivity (Scheme 1).2
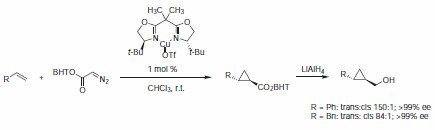
Scheme 1
In a related experiment, Evans announced the first catalytic enantioselective aziridination of styrene.4 Two years later, he reported on an extension of this preliminary work in a full communication (Scheme 2). Using BOX ligand (Product No. 405000), complexed to Cu(I)OTf, in the catalytic enantioselective aziridination of styrene, the BOX ligand having phenyl substituents proved to be superior to the one bearing sterically demanding t-Bu groups (Scheme 2).
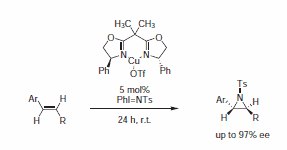
Scheme 2
Reiser et al. discovered a general route to disubstituted γ-butyrolactones. In an Cu(I)-catalyzed asymmetric cyclopropanation of furans with diazo esters, bis(4‑isopropyloxazoline) (Product No. 680192) was used as chiral ligand (Scheme 3). This transformation was performed on 50–100 g scale without a detectable loss of enantioselectivity.5
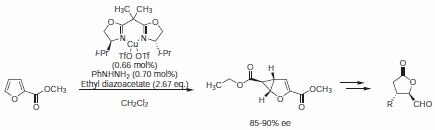
Scheme 3
In his 1991 Journal of American Chemical Society communication, Corey used BOX ligand (Product No. 405000) in an Fe(III)-catalyzed enantioselective Diels–Alder reaction (Scheme 4).3
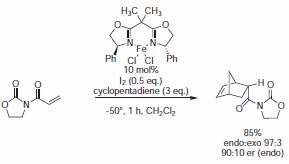
Scheme 4
Two year later, Evans described the Cu(II)-catalyzed version of the enantioselective Diels–Alder reaction of unsubstituted acrylimides using BOX ligand (Product No. 406147) as a chiral Lewis acid.6 The reaction proceeded in good yield exhibiting excellent enantioselectivity for the endo diastereoisomer (Scheme 5). From further investigations by Evans et al., it appears that the best combination is BOX (Product No. 406147) and Cu(II), and the best counterions are OTf and SbF6.1
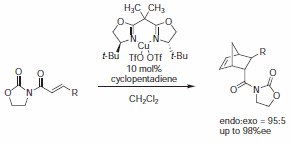
Scheme 5
4‑Aryl- and 4‑alkylsubstituted BOX-based catalysts were widely used in a large number of reactions1 and their behavior can provide a good representation of their efficiency. But BOX ligands with other structural motifs have been successfully used too. BOX ligand (Product No. 405981) has been used in the Cu(I)-catalyzed highly enantioselective Mannich reactions to efficiently produce highly functionalized 4‑oxo-glutamic ester derivatives (Scheme 6).7
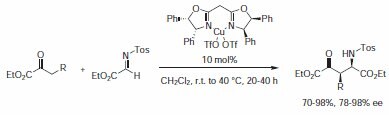
Scheme 6
Ma et al. reported on the enantioselective CuI/bis(oxazoline)- catalyzed addition of propiolates and terminal ynones to 1‑acylpyridinium salt to afford highly functionalized dihydropyridines with excellent enantioselectivity using Product No. 464155 (Scheme 7).8 It is found that the carbonyl group adjacent to the alkyne moiety is essential for the enantioselectivity of the addition.
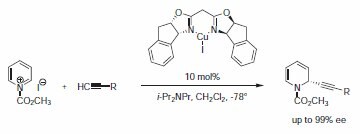
Scheme 7
References
계속 읽으시려면 로그인하거나 계정을 생성하세요.
계정이 없으십니까?