Oxidative stress is implicated in the pathogenesis of lipotoxicity in both animal and human studies. Chronic oxidative stress is linked to insulin resistance in multiple tissues. Oxidative stress is mediated, in part, by reactive oxygen species produced by multiple cellular processes and controlled by cellular antioxidant mechanisms such as enzymatic scavengers or antioxidant modulators. When ROS production exceeds the cellular antioxidant capacity, oxidative damage to cellular components such as proteins, lipids, and DNA occurs. Multiple pathways can contribute to cellular ROS formation, including the mitochondrial electron transport chain (ETC), inflammatory signaling, and endoplasmic reticulum stress.
The ETC is the major source of endogenous superoxide production in the cell. As electrons pass through the ETC, a small fraction escape and prematurely react with molecular oxygen resulting in the production of superoxide. An excess of nutrients will theoretically increase electron flux through the ETC with a resulting increase in the production of superoxide. Excessive ROS production may induce insulin resistance by the activation of stress-activated signaling cascades or by causing damage to mitochondrial proteins and DNA.
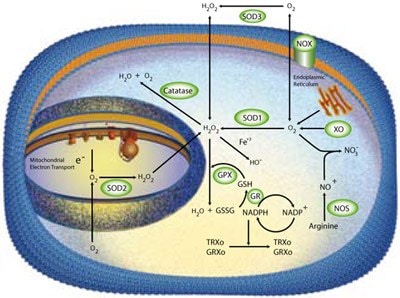
Figure 1.Multiple enzymatic scavengers are utilized by the cell to limit damage from reactive oxygen species. These scavengers include members of the superoxide dismutase (SOD) family, catalase, and glutathione peroxidase.
| Name | Description | Catalog No. |
|---|---|---|
| Catalase from bovine liver | Catalase activates the decomposition of hydrogen peroxide, a reactive oxygen species, into water and oxygen. It functions as a natural antioxidant, protecting cells against oxidative damage to proteins, lipids and nucleic acids. Catalase has also been used to study the role reactive oxygen species play in gene expression and apoptosis. | C9322 |
| Catalase from human erythrocytes | Catalase activates the decomposition of hydrogen peroxide, a reactive oxygen species, into water and oxygen. It functions as a natural antioxidant, protecting cells against oxidative damage to proteins, lipids and nucleic acids. Catalase has also been used to study the role reactive oxygen species play in gene expression and apoptosis. | C3556 |
| Glutathione Peroxidase from bovine erythrocytes | – | G6137 |
| Glutathione Peroxidase from human erythrocytes | – | G4013 |
| Nitric Oxide Synthase, Inducible from mouse | NOS is responsible for the biosynthesis of nitric oxide from L-arginine. iNOS is not calcium/calmodulin dependent and has a Km = 16 µM for L-arginine. | N2783 |
| Peroxiredoxin I Active human | Human Peroxiredoxin I (GenBank Accession No. NM_005614), full length, with N-terminal HIS6 tag, MW = 23 kDa, expressed in a Baculovirus infected Sf9 cell expression system. | SRP0202 |
| Peroxiredoxin II Active human | Human Peroxiredoxin 2, full length (Genbank accession number NM_005809) with N-terminal HIS tag, MW = 22.7 kDa, expressed in Baculovirus infected Sf9 cell expression system. | SRP0203 |
| Protein Disulfide Isomerase from bovine liver | Facilitates formation of the correct disulfide bonds by promoting rapid reshuffling of disulfide pairings. | P3818 |
| Superoxide Dismutase bovine | S9697 | |
| Superoxide Dismutase from bovine erythrocytes | Catalyzes the dismutation of superoxide radicals to hydrogen peroxide and molecular oxygen. Plays a critical role in the defense of cells against the toxic effects of oxygen radicals. Competes with nitric oxide (NO) for superoxide anion (which reacts with NO to form peroxynitrite), thereby SOD promotes the activity of NO. SOD has also been shown to suppress apoptosis in cultured rat ovarian follicles, neural cell lines, and transgenic mice. | S7571 |
| Superoxide Dismutase from human erythrocytes | Catalyzes the dismutation of superoxide radicals to hydrogen peroxide and molecular oxygen. Plays a critical role in the defense of cells against the toxic effects of oxygen radicals. Competes with nitric oxide (NO) for superoxide anion (which reacts with NO to form peroxynitrite), thereby SOD promotes the activity of NO. SOD has also been shown to suppress apoptosis in cultured rat ovarian follicles, neural cell lines, and transgenic mice. | S9636 |
| Thioredoxin Reductase from rat liver | Thioredoxin reductase (TrxR) is a NADPH-dependent oxidoreductase containing one FAD per subunit that reduces the active site disulfide in oxidized thioredoxin (Trx). | T9698 |
| Name | Description | Catalog No. |
|---|---|---|
| Ceramide from bovine brain | – | 22244 |
| Dexamethasone | An anti-inflammatory glucocorticoid with a range of effects on cell survival, cell signaling and gene expression. Use to study apoptosis, cell signaling pathways and gene expression. Glucocorticoid antiinflammatory agent. | D4902 |
| 2',7'-Dichlorofluorescin diacetate | Cell-permeable probe is de-esterified intracellularly and turns to highly fluorescent 2',7'-dichlorofluorescin upon oxidation. | D6883 |
| Dihydroethidium | Redox indicator. Blue fluorescence until oxidized to ethidium. | D7008 |
| Dihydrorhodamine 123 | – | D1054 |
| 2,4-Dinitrophenol | – | D198501 |
| Flavin adenine dinucleotide disodium salt hydrate | – | F6625 |
| ß-Nicotinamide adenine dinucleotide, reduced disodium salt hydrate | – | N8129 |
| ß-Nicotinamide adenine dinucleotide, reduced disodium salt hydrate | – | N1161 |
| Sodium azide | – | 71289 |

Figure 2.Free radicals, such as reactive oxygen species, cause cellular damage via cellular "rusting".
| Name | Description | Catalog No. |
|---|---|---|
| N-Acetyl-l-cysteine | Antioxidant and mucolytic agent. Increases cellular pools of free radical scavengers. Reported to prevent apoptosis in neuronal cells but induce apoptosis in smooth muscle cells. Inhibits HIV replication. May serve as a substrate for microsomal glutathione transferase. | A9165 |
| Aminoguanidine hemisulfate salt | Inhibits both constitutive and inducible nitric oxide synthetase. | A7009 |
| L-Glutathione reduced | Endogenous antioxidant that plays a major role in reducing reactive oxygen species formed during cellular metabolism and the respiratory burst. Glutathione- S-transferase catalyzes the formation of glutathione thioethers with xenobiotics, leukotrienes, and other molecules that have an electrophilic center. Glutathione also forms disulfide bonds with cysteine residues in proteins. Via these mechanisms, it can have the paradoxical effect of reducing the efficacy of anti-cancer agents. | G4251 |
| (±)-α-Lipoic acid | Antioxidant and coenzyme needed for the activity of enzyme complexes such as pyruvate dehydrogenase and glycine decarboxylase. | T1395 |
| Lipoic acid, reduced | – | T8260 |
| Nicotinamide | – | N0636 |
계속 읽으시려면 로그인하거나 계정을 생성하세요.
계정이 없으십니까?