Difluoroacetic Acid as an Effective Mobile Phase Modifier for the LC-UV/MS Analysis of Proteins
Maricar Dube, Analytical Sciences Liaison, Cory Muraco, Global Franchise Manager, Liquid Chromatography Technology, Daniel Weibel, Global Product Manager Solvents
Merck
Abstract
Trifluoroacetic acid (TFA) is typically the mobile phase modifier used for protein analysis by reversed- phase high performance liquid chromatography (RP HPLC), especially when combined with UV detection. TFA improves retention, peak shape, and due to the latter the sensitivity. However, TFA also causes ion suppression in mass spectrometry (MS), thereby significantly reducing the sensitivity. Formic acid (FA) is generally the modifier of choice with MS detection as it provides efficient ionization. However, the use of formic acid results in less efficient chromatographic separation. Difluoroacetic acid (DFA) is a suitable alternative to TFA and FA in the LC-UV/MS analysis of proteins, allowing adequate separation efficiency when compared to FA, and better MS compatibility when compared to TFA.
Introduction
The growth of protein-based therapies and the field of proteomics have propelled technical advancements in the analysis of proteins. Characterization of these large and complex molecules utilize a range of techniques - chromatography being one of them. The analysis of intact proteins and protein digests by reversed-phase high performance liquid chromatography (RP-HPLC) provides researchers with valuable information not only about a protein’s identity, but also the post-translational modifications that affect its properties.
During the analysis of proteins by RP-HPLC, trifluoroacetic acid (TFA) is added to the mobile phase to obtain sharp and symmetrical peaks with adequate retention,1 particularly when used in conjunction with UV detection. The added TFA,
(1) lowers the pH of the mobile phase to well below the pKa of side-chain carboxyls, thereby facilitating maximum retention of acidic moieties, and
(2) acts as an effective ion-pairing agent2 for the basic moieties of the protein. In the absence of ion pairing reagents, the residual silanols in the HPLC column interact with the protein analytes, causing band broadening, which in turn leads to reduced efficiency and sensitivity.
HPLC coupled to a mass spectrometer (specifically electrospray ionization (ESI)) has become a widely used technique in protein analysis. The mass spectrometer offers mass selectivity and higher sensitivity compared to UV, giving researchers more valuable information about analyzed protein(s). However, TFA is not compatible with MS; it suppresses signal intensity because of ion-pair formation, high conductivity and surface tension.1 TFA also leaves background in the mass spectrometer that is difficult to remove.3 This signal or ion suppression in turn compromises the sensitivity of the technique.
Because of TFA’s incompatibility with LC-MS, investigators often use formic acid (FA) when conducting protein separations with MS detection.4 Unfortunately, separations with FA as a modifier often result in poor peak shape and less efficient separations.3 This result is because FA is a poorer ion- pairing agent and also a weaker acid compared to TFA.
This article explores the use of difluoroacetic acid (DFA) as an attractive acid modifier alternative in the LC-UV/ MS analysis of proteins. DFA is a strong ion-pairing agent and provides the desired low pH (it has a lower pKa compared to FA).5 Table 1 shows the structures and pKa’s of TFA, FA and DFA.
Experimental Conditions
The HPLC analyses were carried out using a Shimadzu Nexera LC system equipped with UV and MS detectors. The flow was split between detectors to minimize band broadening from the sample plug having to pass through multiple detectors in line. The conditions are listed in Table 2.
Results and Discussion
The combination of high performance liquid chromatography with mass spectrometry (LC-MS) has become a critical tool in protein analysis. Traditionally, TFA is the mobile phase additive used as it provides adequate retention of proteins, and also gives sharp and symmetrical peaks with UV detection (Figure 1A). However, it is not recommended for MS detection as it suppresses the analyte signal (Figure 1B).
To get better MS signals, investigators use FA because it gives better ionization efficiency of the analytes (Figure 2B). Unfortunately, FA as a mobile phase modifier often results in poor peak shape and inefficient separations (Figure 2A).
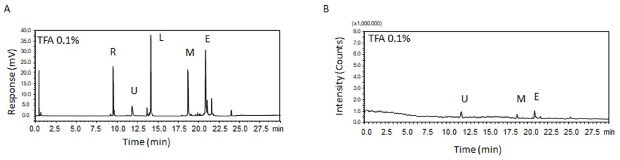
Figure 1.(A) UV and (B) MS chromatograms of five proteins when using TFA as modifier. R – Ribonuclease, U – Ubiquitin, L – Lysozyme, M – apo-Myoglobin, E – Enolase

Figure 2.(A) UV and (B) MS chromatograms of five proteins with FA as modifier. R – Ribonuclease, U – Ubiquitin, L – Lysozyme, M – apo-Myoglobin, E – Enolase

Figure 3.(A) UV and (B) MS chromatograms of five proteins with DFA as modifier. R – Ribonuclease, U – Ubiquitin, L – Lysozyme, M – apo-Myoglobin, E – Enolase
A combination of UV and MS detectors provides investigators with much richer information than just a UV detector, although using both the detectors in line will contribute to band broadening in the MS and must be considered. Using TFA to benefit UV detection does not provide any useful MS data. Using FA to benefit MS detection sacrifices separation efficiency. Therefore, there is a need to find a suitable, MS-friendly solvent additive for the HPLC analysis of proteins that will provide efficient separation without sacrificing on MS signal because of ion suppression. DFA is a viable candidate. This reagent is a stronger acid than FA and does not suppress ionization as much as TFA. Figure 3 shows the UV and MS chromatograms of five proteins with DFA as the mobile phase modifier.
Comparing the UV chromatograms in Figures 1A, 2A, and 3A illustrates the ion pairing strength of TFA, FA and DFA. TFA provides the strongest ion pairing strength, affording the strongest retention of the proteins, followed by DFA. FA is the poorest ion pairing reagent of the three.
To further explore how the different modifiers influence separation efficiency, a separate experiment was carried out where the concentrations of TFA, FA and DFA were kept the same at 10 mM. Peak widths at half height (W1/2) were measured for each of the analyte peaks, as peak width at half height can be used as a measure of efficiency during gradient LC assays. Smaller values correspond to narrower peak width, indicating better efficiency. The results are shown in Figure 4.
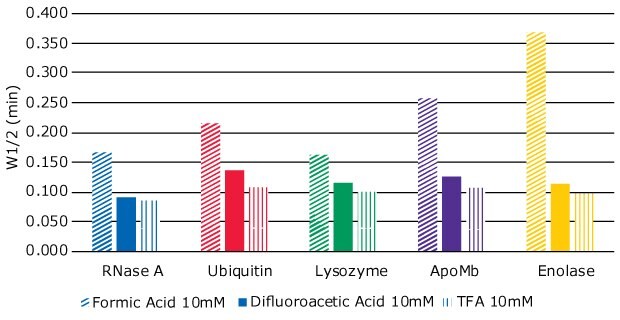
Figure 4.Peak width at half height (W1/2) of five proteins, using three different mobile phase modifiers – formic acid (FA), difluoroacetic acid (DFA), and trifluoroacetic acid (TFA)
TFA gave the narrowest peak widths, indicating better efficiency, followed closely by DFA. FA as the modifier gave the least efficient separation.
The influence of the three modifiers on the MS response can be seen by comparing Figures 1B, 2B, and 3B. Clearly, the addition of FA as modifier resulted in strong MS signals, but at the cost of separation efficiency. DFA gave better signals than TFA. This observation is also illustrated in Figure 5A where signals from each analyte for each of the modifiers are plotted against the total ion current (TIC). Figure 5B is an exploded view of just DFA and TFA MS signals, for better comparison.
As seen in Figure 5B, as well as Figure 1B, MS signals from TFA are strongly suppressed. In fact, the proteins RNase A and Lysozyme were not detected in MS. The use of DFA as modifier gave detectable MS signals as shown in Figure 3B.
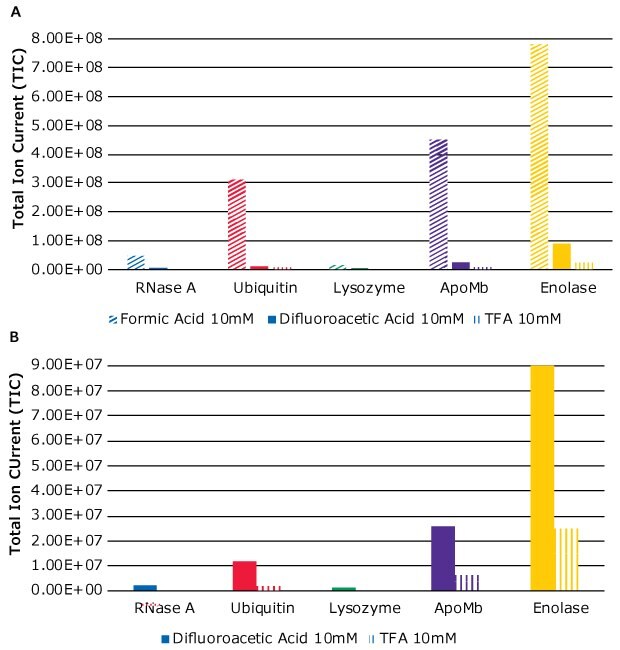
Figure 5.(A) MS signals when using three different modifiers – FA, DFA, and TFA. (B) Exploded view of MS signals when using DFA and TFA as modifiers.
Conclusion
In the HPLC analysis of proteins where combining UV and MS detectors is sometimes necessary, DFA is an attractive alternative to TFA and FA as mobile phase modifier. This reagent provides better separation efficiency than FA, and it does not cause strong ion suppression like TFA. However, DFA causes reduced MS ion yield compared to FA and slightly broader peaks than obtained with TFA. Therefore, users need to do an assessment regarding a possible and acceptable compromise for their application.
References
계속 읽으시려면 로그인하거나 계정을 생성하세요.
계정이 없으십니까?