Hematopoietic Stem Cell Culture Protocols
Hematopoietic stem cells residing within mouse bone-marrow were first discovered by Till and McCulloch in the 1960s and indicated that (1) hematopoiesis could be studied as a quantitative science, (2) clonal hematopoietic cells in the marrow existed that could give rise to mixed myeloid progeny (granulocytes, macrophages, red cells, megakaryocytes), (3) some of these cells could self-renew and (4) in the spleens of these mice, cells existed that could also make lymphocytes. All colony-forming activity of human bone marrow (BM) cells is found in the CD34+ progenitor cell fraction. Clinical transplantation studies that used enriched CD34+ cells from bone-marrow, umbilical cord and peripheral blood mononuclear cells (PBMCs) indicated the presence of HSCs with long-term tissue reconstitution ability. Below are commonly used in vitro cell culture protocols and assays used to isolate, expand, differentiate and characterize human CD34+ hematopoietic stem cell populations from various tissues.
Isolation of Human CD34+ Hematopoietic Progenitor Cells
HSCs can be isolated using flow cytometry based on surface marker expression. Primitive multipotent hematopoietic progenitors have phenotypes with CD34+/CD38-/CD45RA-/CD71- expression. Other positive markers for hematopoietic progenitors include CD133+, CD90+ (Thy-1), ALDH+ and Sca-1+. Other negative marker for hematopoietic progenitors include mature blood lineage (Lin-) markers: CD2-, CD3-, CD19-, CD41-, CD16-, CD14-, and CD15-. The ideal sample is fresh, anticoagulated blood or tissue samples. The preparation details vary, depending on the specific tissue source. If samples cannot be processed within 48 hours, they should be frozen.
- Dilute samples 1:1 in D-PBS without Mg2+ or Ca2+ (D8537).
- Pour 20 mL Ficoll (F5415) into a 50-mL tube and slowly layer (tilting tube and running the cells down the side of the tube) 25 mL of diluted blood or marrow on top.
- Centrifuge at room temperature 1100g for 20 min.
- Remove half of the top layer, and discard.
- Carefully pipet off “cloudy” interface layer (~10 mL) and transfer into a clean 50-mL tube. Wash these cells with 50 mL PBS without Mg2+ and Ca2+ (D8537).
- Resuspend cells in media with serum or protein (D-PBS or Hank’s with 2–6% FBS or HSA).
- Centrifuge cells, and wash twice in D-PBS without Mg2+ and Ca2+ (D8537).
- Lyse and remove RBCs by resuspending in cold NH4Cl (A9434) solution at 3–4 times the original sample volume.
- Incubate on ice for 10 min.
- Centrifuge cells, and wash twice in D-PBS without Mg2+ and Ca2+ (D8537). Resuspend cells in media with serum or protein (D-PBS or Hank’s with 2–6% FBS or HSA).
- Resuspend cells in Hank’s + FBS at 107/mL.
- Remove samples of cells for controls (~105 cells/tube) as follows: Unstained cells; Irrelevant antibody controls for FITC, phycoerythrin, and Cy5; Single-color positive controls for FITC, phycoerythrin, and Cy5.
- To the remaining cells, add the appropriate antibodies for the chosen procedure. Incubate cells for 30 min at 4 °C.
- Wash cells twice and resuspend in Hank’s + FBS containing 2 µg/mL propidium iodide (PI) (P4170). Cells are now ready for sorting.
- Before the fluidic system is turned on, remove the fluid filter and use a syringe and blunt needle to inject a sufficient volume of filtered 10% bleach (425044) to flood the sample line.
- Remove the syringe, attach a new, clean filter, and reassemble the fluidic system.
- Turn on the fluidic system and run a sample tube of filtered 10% bleach for approx. 10 min.
- Remove the bleach tube and back flush the sample line with sterile sheath fluid.
- Rinse the outside of the sample line with alcohol, and then with sterile D-PBS. Cells are gated on forward- and side-scatter and PI staining (PI-viable cells). Cells are then gated for high expression of CD34 and low expression of CD45, CD38 and CD71.

Figure 1. Human hematopoietic stem cell markers analyzed by flow cytometry. Flow cytometric characterization of the bench-scale HSC expansion from a variety of donors reveals that the Stemline™ Hematopoietic Stem Cell Expansion Medium generates a significant expansion of both committed (Blue, CD34-/CD15+/CD41+) and early (Red, CD34+/CD15-/CD41-) hematopoietic progenitor cells.
Expansion of Human CD34+ Hematopoietic Progenitor Cells
- Prepare cell culture media. Combine the Basal Medium and the Supplement Mix of the PromoCell HPC Expansion Medium DXF (C-28021) according to the instructions. Then, add an appropriate amount of PromoCell Cytokine Mix E (C-39890/C-39891) to obtain the completely supplemented Expansion Medium. Pre-equilibrate an appropriate amount of the supplemented medium in the incubator at 37 °C and 5% CO2 for 30 minutes.
- Plate cells. Freshly isolated HPCs: Plate the HPCs in the pre-equilibrated medium at a density of 5X103 cells/ml. Cryopreserved HPCs: Thaw the cells for 2 minutes in a water bath. After thawing, immediately transfer them into the pre-equilibrated medium at a density of 5,000 cells per ml. Use at least 9 ml of medium per vial of cryopreserved cells.
- Incubate the cells for 4 days at 37 °C and 5% CO2. For a partial medium change, remove the cells from the incubator. To create a single cell suspension pipet up and down several times and transfer the whole content of the tissue culture vessel into a 50 ml conical tube. Spin the cells down for 10 min at 240 x g. Then, carefully aspirate the upper two thirds of the medium. Gently resuspend the cells in the remaining third of the medium and replenish to the original volume with fresh cytokine-supplemented HPC Expansion Medium DXF (C-28021). Incubate for another 6–8 days to allow sufficient expansion of the cells. Replace two thirds of the medium as described above every 3 days.
- Harvest cells by collecting the medium from the tissue culture vessel containing the expanded HPCs. Pipet up and down several times in order to release loosely attached cells and to obtain a single cell suspension. Spin down the harvested HPCs at 240 x g for 10 minutes and discard the supernatant.
- Resuspend the cells in PromoCell HPCs Expansion Medium DXF (C-28021) and count them. The HPCs are now ready to be used in your experiments.

Figure 2. Morphology of human CD34+ hematopoietic progenitor cells. Suspension culture of bone-marrow derived isolated human CD34+/CD38-hematopoietic stem cells (A, 10X and B, 40X).
The Colony Formation Unit (CFU) Cell Assay
The colony forming unit (CFU) cell assay, or CFC assay, is used to study the proliferation and differentiation of hematopoietic progenitors by their ability to form colonies in a semisolid medium such as methylcellulose or agar. The number and the morphology of the colonies formed by a fixed number of input cells provide preliminary information about the ability of progenitors to differentiate and proliferate. This assay is useful for assessing myeloid (granulocytic, monocytic, erythroid, and megakaryocytic cells) but not lymphoid lineage differentiation.
- Thaw a vial of frozen CD34+ hematopoietic stem cells (C-12921) quickly at 37 °C or use freshly isolated cells.
- Take 3X103 cells into a sterile microtube containing cold 2% FBS/IMDM. Pre-adjust the volume of 2% FBS/IMDM so that the final suspension volume will be approximately 0.3 mL.
- Suspend the cells and transfer the entire 0.3 mL cell suspension to a 3 mL aliquot of thawed and supplemented Hematopoietic Progenitor Expansion Medium DXF (C-28021). Additional methylcellulose (1%) (M7027) and additional cytokines may be added at this stage to induce lineage differentiation.
- Vortex vigorously and let the tube stand still for 3 min (bubbles should rise to the top).
- Attach a 16 gauge blunt-end needle to a 3 mL syringe and draw up 2.2 mL. Do not draw up large bubbles; expel them at the beginning by pushing out a couple of times. Push out 1.1 mL each into two 30 mm non-treated dish and spread out the mixture evenly by swirling and rotating plate.
- Place duplicate plates in a 100 mm plate together with a water dish containing 3 mL sterile water for additional humidity. Culture for 14-17 days.
- Characterize and score the colonies according to their morphology with an inverted microscope at 40x magnification in a culture dish marked with a scoring grid.
- For further analysis of differentiation and proliferation, cells from the entire CFU assay plate can be recovered by suspending in several volumes of room temperature 2% FBS/IMDM. After centrifugation at 300 x g for 10 min at 4 °C, the cells are resuspended in 2% FBS/IMDM, counted, and either stained with antibodies for flow cytometry or transferred to slides using a Cytospin centrifuge for Giemsa staining.
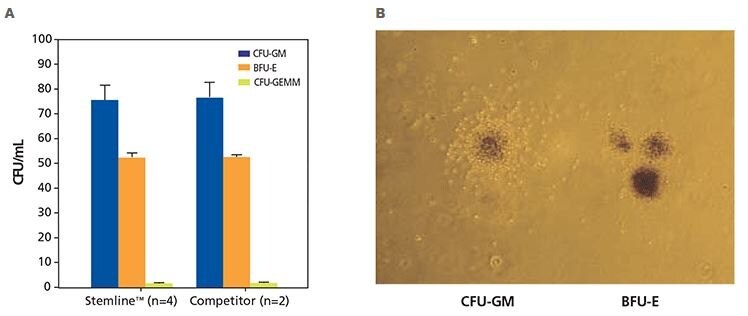
Figure 3. Colony formation unit (CFU) cell assay results of human CD34+ hematopoietic progenitor cells. A) Human CD34+ hematopoietic stem cells isolated from normal, healthy human bone marrow were cultured in either Stemline™ HSC media in methylcellulose with Epo or competitor media in methylcellulose with Epo. Cultures were scored after 12 - 16 days of culture using an inverted microscope. Cultures were scored for burst-forming unit-erythroid (BFU-E), colony-forming unit-granulocyte, -monocyte (CFU-GM) and colony-forming unit-granulocyte, - erythrocyte, -macrophage, and megakaryocyte CFU-GEMM). B) Differentiated human CD34+ hematopoietic progenitor cells showing a human granulopoietic colony containing clonogenic precursors of granulocytes and macrophages (CFU-GM) and human erythroid progenitor colony (BFU-E). The CFU-GM colonies demonstrate a relatively homogeneous morphology and typically have a concentrated central core of cells surrounded by a less dense halo of cells. The BFU-E colonies are frequently referred to as having a grape-like morphology and range in size from a single, large cluster containing several hundred erythroblasts to 14 or more clusters containing thousands of cells (40X magnification).
계속 읽으시려면 로그인하거나 계정을 생성하세요.
계정이 없으십니까?