Protocol Guide: Differentiation of Human Neural Stem Cells using TrueGel3D® HTS Hydrogel Plates
Recently, 3D cell culture neural disease models have become attractive alternatives to animal models and traditional 2D cell cultures. Co-cultures of neurons, astrocytes and oligodendrocytes have been shown to recapitulate many in vivo CNS phenotypes including neural synaptic complexity, gene expression patterns and neuroinflammatory responses. However, these models often rely on undefined animal derived hydrogels which are not amendable to high throughput screening applications. The TrueGel3D® HTS Hydrogel Plate is a ready-to-use solution to easily establish 3D cell cultures using fully synthetic hydrogels in a simple and automation-compatible manner. Here we demonstrate how ReNcell® VM human neural stem cells can be differentiated into β-tubulin expressing neurons using TrueGel3D® HTS hydrogel plates following an optimized step-by-step culture protocol.
Neural Stem Cell Protocol
Expansion and Differentiation of Human NSCs
ReNcell® VM Human Neural Progenitor Cell Line (SCC008) can be expanded on laminin (CC095-M) coated (20 µg/mL) tissue culture plastic first and then seeded in TrueGel3D® HTS Hydrogel Plates (TRUE-HTS1, TRUE-HTS10). Alternatively, cells can be thawed and directly seeded in TrueGel3D® HTS Hydrogel Plate. Ensure cells are at high viability before seeding into TrueGel3D® HTS Hydrogel Plates. The cells should be handled and stored according to the manufacturer’s instructions. All steps should be performed inside a laminar flow hood to ensure sterility.
- Preparation of culture media:
- Add 200 µl/well of the ReNcell® VM cell suspension in ReNcell® expansion media at a density of 60,000 cells/well to the TrueGel3d HTS hydrogel plates.
- Change medium every 2-3 days.
- Track the culture development using a transmission light microscope. Note: Immediately after seeding, RenCells® will appear round on top of the hydrogel. Within 3 days of expansion, the cells will duplicate and start to migrate forming clusters mainly on top of the gel.
- To induce differentiation, change media from ReNcell® expansion to differentiation media and monitor over 14 days exchanging media every 2-3 days.
Note: After 14 days of differentiation the culture will look like a neural network: the initial clusters will appear to stay on top of the gels connecting one other, but the space between these islands will be occupied by a thick 3D neuronal network. Cultures can be continued at least up to 25 days without noticing regression; for longer times, it is important to increase the thickness and depth of the 3D neuronal network.
Antibody Characterization by Microscopy
- Aspirate the culture medium.
- Fix the cells with 4% formaldehyde solution (100496) for 30 min at room temperature.
Note: for best results, especially in case of 2D controls, prepare a 4% formaldehyde solution in ReNcell® NSC Maintenance Media (SCM005). - Wash three times with PBS (D8537) (200 µl/well) for 5-10 min each.
Note: the plates can be closed again with the sealing foil and stored at 4 °C for future use - Block for 2 hours at room temperature using:
- Incubate overnight at 4 °C with 150 µl/well of the appropriate primary antibody diluted accordingly in the corresponding blocking buffer. For example: Rabbit Anti-β-Tubulin III IgG (T2200) can be used at a 1:100 dilution in permeable blocking buffer.
- Wash three times with PBS (D8537) (200 µl/well) for 5-10 min each.
- Incubate for 2 hours at room temperature with 200 µl/well of the appropriate fluorescently labelled secondary antibody diluted accordingly in the same blocking buffer. For example: Atto 633 anti-rabbit IgG (41176) can be used at a 1:200 dilution in permeable blocking buffer.
Note: Hoechst 3358 (94403) counterstaining (1:500 dilution in PBS) can be added together with the secondary antibody to counterstain the cell nuclei (150 µl/well). - Wash three times with PBS (D8537) (200 µl/well) for 5-10 min each.
- Image: Bright field and fluorescence images showing the neural network spanning the entire depth of the hydrogel can be acquired by an inverted epifluorescence or confocal fluorescence microscope equipped with a 20–30X objective.
Results
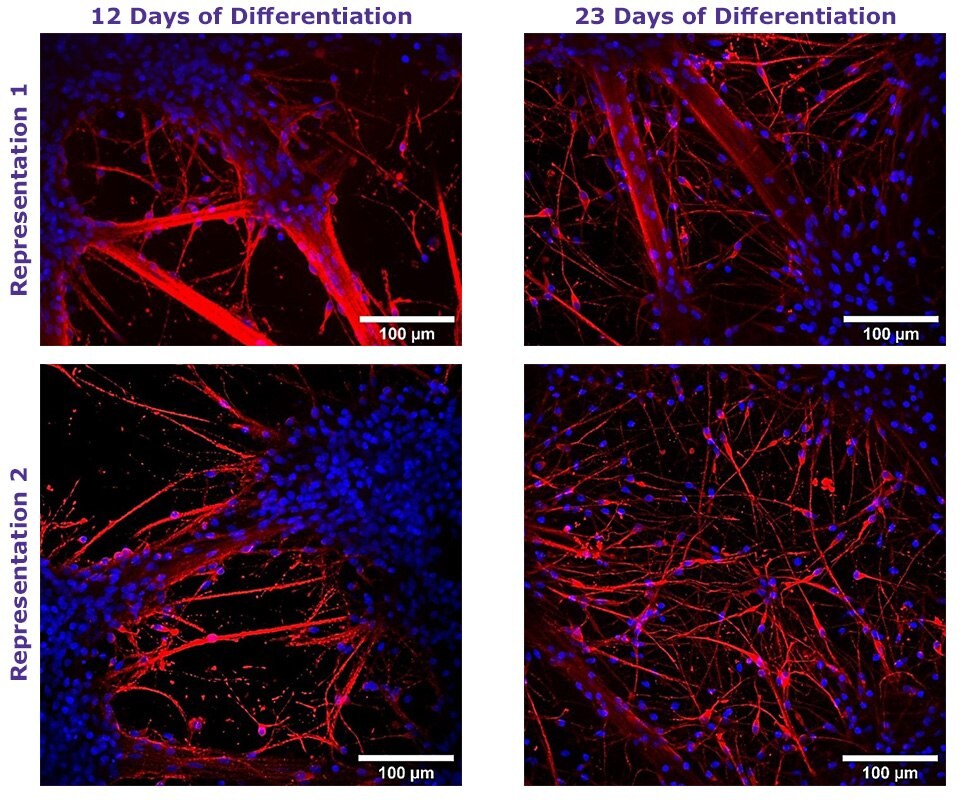
Figure 1. Neural Differentiation of Human NSCs using TrueGel3D® HTS Hydrogel Plates.
Fluorescent staining of day 12 and 23 neural differentiated ReNcell® VM NSC cultured in 3D using TrueGel3D® HTS hydrogel plates acquired with an Olympus SpinSR10 spinning disk confocal microscope. Neurons express β-Tubulin III (Red) and counterstained with Hoechst 3358 (Blue).
계속 읽으시려면 로그인하거나 계정을 생성하세요.
계정이 없으십니까?