Generating Organotypic 3D Skin Cultures as an in-vitro Model of the Human Epidermis
Skin is the largest organ in the human body and serves numerous functions including protection, the absorption of substances, and thermoregulation. Skin has a complex stratified structure consisting of three main layers: 1) the tightly packed cells of the epidermis form a barrier against intruders and water loss, and includes an outer layer called the stratum corneum, consisting of overlapping, nonviable corneocytes that protect against threats such as UV radiation and pathogens; 2) the dermis, which supports the epidermis and contains nerve endings, sweat glands, sebaceous glands, hair follicles, and blood vessels. The basal layer of the dermis is where keratinocytes constantly undergo mitosis to replenish cells; and 3) the subcutaneous layer, or hypodermis, which contains mostly adipocytes and acts to protect underlying tissues, as an energy reserve, and to provide some thermoregulation via insulation.
Historically, animal models have been used to test the toxicity and efficacy of cosmetics1 and transdermal drug absorption.2 Due to ethical concerns, however, these animal models have gradually been replaced by “cruelty-free” in vitro organotypic skin models that use primary human cells and cell culture inserts to recapitulate the stratified epidermal architecture.3 In 2013, the European union was the first to ban animal testing of cosmetics entirely (Cruelty Free International). Here, we describe a step-by-step culture protocol that can be used to generate human epidermal skin models using primary human keratinocytes (PHK), dermal fibroblasts (primary human fibroblasts, PHF), collagen-coated MilliCell® culture inserts and the proprietary 3dGRO™ Skin Differentiation Medium which supports the robust differentiation of keratinocytes during the air:liquid interface culture steps.

Figure 1.Organotypic human skin culture protocol overview.
Skin cell culture protocol
Important note:The quality of the primary human keratinocytes (PHKs) and fibroblasts (PHFs) is critical to generating high quality 3D skin cultures. Use only primary cells that are at passage 1 or 2, and do not allow cells to become over-confluent.
- Thaw one vial of primary human keratinocytes (C-12001, C-12005, 102-05N) and human dermal fibroblasts (C-12300, 106-05N) or NIH 3T3 cells (93061524) in appropriate growth media (C-20111, 131-500, C-23110, 116-500). Refresh with fresh media every other day until the culture reaches 80 – 90% confluence.
- Prepare collagen/PHF beds (prepare on ice) (8 mL total volume)
a. Add 6 mL rat tail collagen (08-115) into 15 mL conical tube (see lot-specific datasheet for concentration).
b. Add 1.6 mL of 5X reconstitution buffer (1.1% NaHCO3, 0.025N NaOH, 100 mM HEPES, 5X DMEM/F12).
Mix well. Avoid introducing bubbles into the collagen.
c. Add 400 µl PHFs (4x106 cells/mL cell suspension) or NIH 3T3 cells. Mix thoroughly but avoid introducing
bubbles to collagen. Keep on ice.
d. Aliquot 400 µL of the collagen/PHF mixture directly to the center of each MilliCell insert (MCHT12H48).
Avoid introducing bubbles while aliquoting. Incubate the inserts/plates at 37 °C for at least 30 minutes to allow
complete polymerization. After gelation, collagen/PHF beds remain usable for up to five hours without adding
any medium when maintained at 37 °C. - Add 0.5 mL of the PHKs (4x105 cells/mL cell suspension) to each collagen/PHF bed. Be careful to not damage the collagen/PHF bed as this may cause leakage or contraction. Add 2 mL of keratinocyte media to the outside of the inserts in a regular 12-well plate. Incubate at 37 °C overnight. The next day, without removing the original medium, add an additional 0.5 mL of keratinocyte media to the inside of each insert and incubate at 37 °C for 2 more days. PHKs should be in submerged culture on collagen beds for a total of three days. High quality PHKs will approach 100% confluence by the third day of submerged culture.
- On the 4th day, gently aspirate the media from each insert. Do not touch or damage the surface of the collagen bed. Use sterile forceps to transfer the inserts to a deep-well 12-well plate (Greiner Bio-One 665110) containing 4.5 mL of 3dGRO™ Skin Differentiation Medium (SCM310) in each well.
Note: You must add the exact amount of medium to the outside of the inserts to allow the skin cultures to be maintained at the air:liquid interface. If too much media is added to the outside of the insert, this may force extra media to enter the insert and soak the skin surface, resulting in disruption of differentiation. - Incubate the skin culture at 37 °C for an additional 10 days. During the 10 day incubation, change the 3dGRO™ Skin Differentiation Medium every other day and remove any bubbles from beneath the insert membrane. After 10 days of 3D skin culture, approximately 8 to 10 layers of live epithelium should be produced.
Results

In-Vitro Organotypic Skin Culture (A)
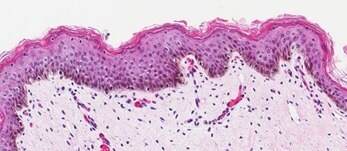
Neonatal Skin Tissue (B)

(C)
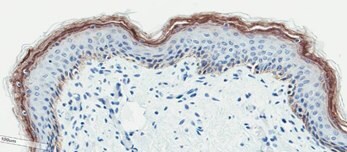
(D)
Figure 2. Organotypic skin models have stratified epithelial morphology comparable to neonatal skin tissue. H&E staining of in vitro skin culture and ex vivo skin sections containing both dermal and epidermal stratified layers (A, B). Filaggrin staining identifies cornified keratinocyte layers (brown) (C, D).

A

B

C
Figure 3. Organotypic skin models contain actively proliferating keratinocytes. BrdU (Bromodeoxyuridine/5-bromo-2'-deoxyuridine) is an analog of the nucleoside thymidine and can be used in assays to identify proliferating cells using BrdU-specific antibodies. In vitro skin models stained with anti-BrdU antibody (brown) (A), H&E (B), and anti-filaggrin antibody (brown) (C) identify cells proliferating within the basal layer of the dermis.
References
계속 읽으시려면 로그인하거나 계정을 생성하세요.
계정이 없으십니까?