Analysis of Adulterated Lemon Essential Oil on the SLB-5ms
Prof. Luigi Mondello
University of Messina, Messina, Italy
Reporter EU Volume 25
Introduction
Citrus essential oils are of high economical importance in many parts of the world. They are complex substances that are used mainly in the food, beverage, cosmetic and perfume industries. They are extracted by means of cold mechanical pressure applied on the fruit peel. The end products are mixtures of more than 200 components that can be grouped into non-volatile (1-15%) and a volatile (85-99%) fractions. The latter contains different classes of compounds (mainly mono and sesquiterpene hydrocarbons, and their oxygenated derivatives, along with aliphatic aldehydes, alcohols and esters), which are present in a wide range of concentrations. Qualitative and quantitative analysis is fundamental for quality control and, often, for the detection of adulterants. The adulteration of these valuable products through the addition of cheaper compounds is, unfortunately, a common fraud.
Citrus Essential Oil Composition
Citrus essential oils differ from each other mainly in their quantitative compositions, both of the volatile and non-volatile fractions. Hence, citrus oils have different olfactory properties that make some oils, such as bergamot or lemon, more valuable than other oils, such as sweet orange. For example, in 1999 in Italy, 1 kg of winter lemon essential oil cost between 12.9 and 16.5 Euro. During this year, Italy accounted for about 20% of the entire world’s production of lemon oil. The same amount of sweet orange oil cost approximately 0.7 Euro in 1999.
Adulteration of Citrus Essential Oils
One of the most common adulterations to mandarin, bergamot, bitter orange, and lemon oils (with the latter probably being the most frequent target), is the addition of low-cost lime or orange oil. Sweet orange oils or sweet orange oil terpenes are characterized by the presence of about 0.1% of δ-3-carene. This monoterpene hydrocarbon is either missing or present at trace levels in other citrus oils. The contrary is true for another monoterpene hydrocarbon, camphene, which is practically absent in sweet orange oils or terpenes, but present at a higher level in lemon oil (about 0.06%). The δ-3-carene level and the δ-3-carene/camphene ratio are particularly useful for the detection of the possible addition of these sweet orange oils or orange oil terpenes.
Analysis of Citrus Essential Oils
Figure 1 shows the Fast GC analysis of pure lemon oil on the SLB-5ms column. The SLB-5ms column was chosen for its ability to resolve the analytes of interest, even under Fast GC conditions (fast oven ramp rate, fast carrier gas linear velocity, narrow bore column). The δ-3-carene/camphene ratio cannot exceed a value of 0.140 for the sample to be considered pure lemon oil. Figure 2 shows the Fast GC analysis of pure sweet orange oil on the SLB-5ms column. A very high δ-3-carene/camphene value was obtained. Figure 3 shows the Fast GC analysis of lemon oil adulterated with 5% sweet orange oil. A δ- 3-carene/camphene value of 0.275 was obtained, which exceeds the maximum value to be considered pure lemon oil.
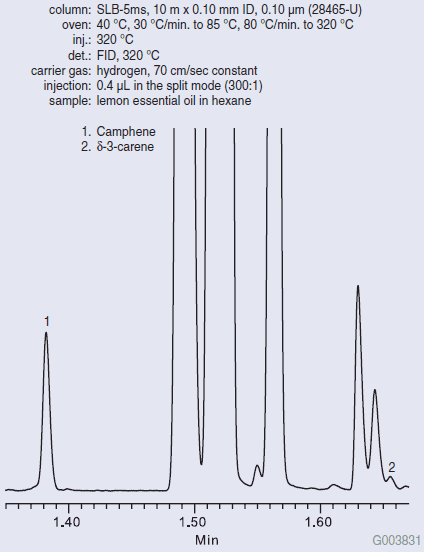
Figure 1.Fast GC Analysis of Pure Lemon Essential Oil
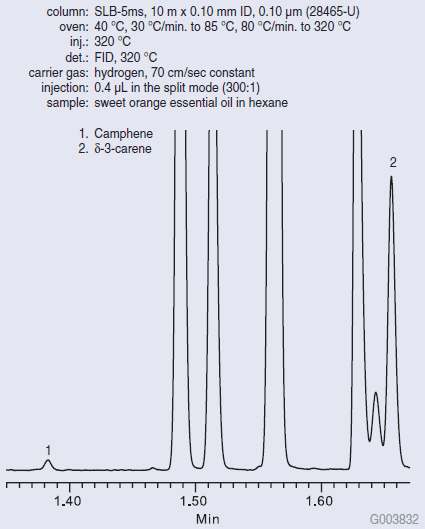
Figure 2.Fast GC Analysis of Pure Sweet Orange Essential Oil
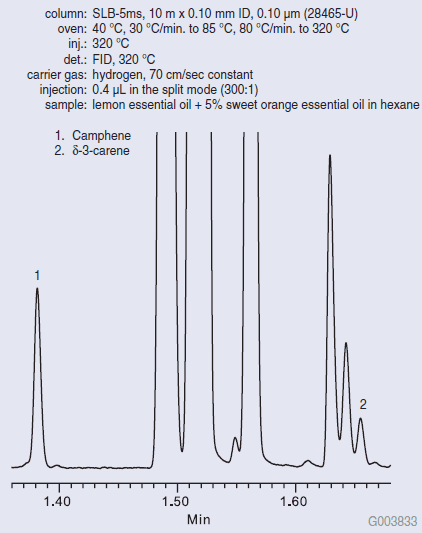
Figure 3.Fast GC Analysis of Adulterated Lemon Essential Oil
Conclusion
There are two unwanted results when expensive citrus oils are diluted. First, the food, beverage, cosmetic, or perfume manufacturer overpays for a raw material. Second, the end consumer receives an inferior product. Through the use of gas chromatography, chemists in these industries have the ability to verify the purity of the citrus essential oils being used in their products. The SLB-5ms column, available in Fast GC dimensions, proved to be a good choice for this application due to its ability to resolve the analytes of interest.
계속 읽으시려면 로그인하거나 계정을 생성하세요.
계정이 없으십니까?