추천 제품
Grade
certified reference material
Agency
BCR®
제조업체/상표
JRC
기술
HPLC: suitable
gas chromatography (GC): suitable
형식
neat
저장 온도
2-8°C
SMILES string
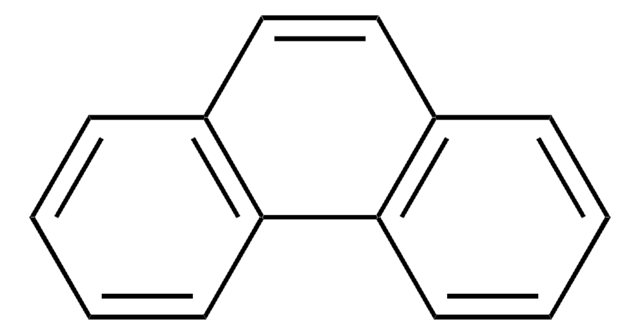
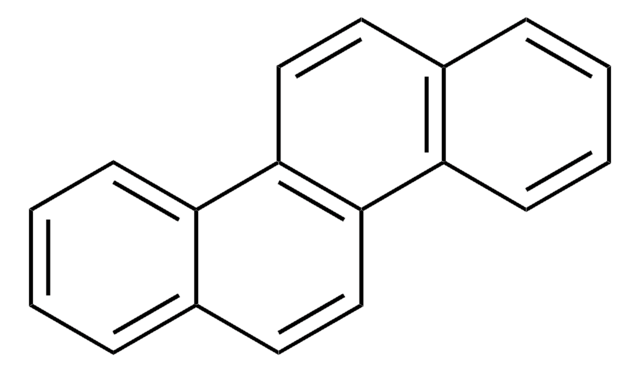
c1ccc2c(c1)ccc3ccc4ccccc4c23
InChI
1S/C18H12/c1-3-7-16-13(5-1)9-11-15-12-10-14-6-2-4-8-17(14)18(15)16/h1-12H
InChI key
TUAHORSUHVUKBD-UHFFFAOYSA-N
분석 메모
For more information please see:
BCR134
BCR134
법적 정보
BCR is a registered trademark of European Commission
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
시험 성적서(COA)
Lot/Batch Number
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact 고객 지원 부서
Min Wu et al.
Frontiers in bioscience : a journal and virtual library, 9, 2807-2818 (2004-09-09)
Remarkably different conformations can result when DNA binds with stereoisomeric compounds containing differing absolute configurations of substituents about chiral carbon atoms. Furthermore, the biochemical functions of covalent adducts with DNA are strongly affected by the stereochemistry of the ligands. Such
I Pontén et al.
Mutagenesis, 16(1), 65-69 (2001-01-05)
The adduct that would arise from cis opening of (+)-(1S,2R,3R, 4S)-3,4-dihydroxy-1,2-epoxy-benzo[c]phenan-threne (benzo[c]phenanthrene diol epoxide-2, where the benzylic hydroxyl group and the epoxide oxygen are trans) by the exocyclic N6-amino group of deoxyadenosine was incorporated at the marked site into four
K K Laali et al.
The Journal of organic chemistry, 66(3), 780-788 (2001-06-30)
The first series of persistent carbocations derived from mono- and disubstituted chrysenes Ch (5- methyl- 3, 2-methoxy- 19, 2-methoxy-11-methyl- 20, 2-methoxy-5-methyl- 21, and 9-methyl-4H-cyclopenta[def]chrysene 22), monosubstituted benzo[c]phenanthrenes BcPh (3-methoxy- 23, 3-hydroxy- 24), and monosubstituted benzo[g]chrysenes BgCh (12-methoxy- 25; 12-hydroxy- 26)
Yazhen Wang et al.
Chemical research in toxicology, 21(7), 1348-1358 (2008-06-14)
The conformation of the 1 R,2 S,3 R,4 S-benzo[ c]phenanthrene- N (2)-dG adduct, arising from trans opening of the (+)-1 S,2 R,3 R,4 S- anti-benzo[ c]phenanthrene diol epoxide, was examined in 5'- d(ATCGC XCGGCATG)-3'.5'-d(CATGCCG CGCGAT)-3', where X = 1 R,2
A Pal et al.
Biochemistry, 40(24), 7047-7053 (2001-06-13)
The molecular basis for catalytic differences between structurally closely related murine class alpha glutathione (GSH) transferases mGSTA1-1 and mGSTA2-2 in the GSH conjugation of anti-diol epoxide isomers of benzo[c]phenanthrene (anti-B[c]PDE) was investigated. GSH conjugation of both (-)- and (+)-enantiomers of
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.![Benzo[a]pyrene ≥96% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/253/820/be96d879-1811-46c0-8f11-612019691c2d/640/be96d879-1811-46c0-8f11-612019691c2d.png)
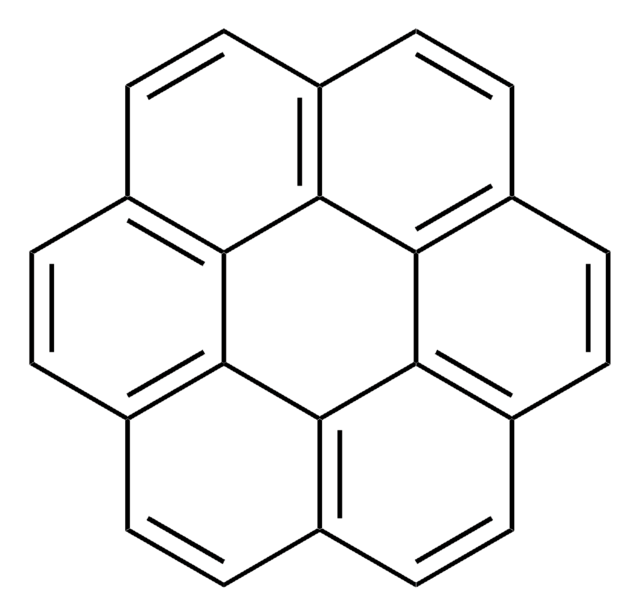
![Dibenzo[a,l]pyrene BCR®, certified reference material](/deepweb/assets/sigmaaldrich/product/structures/937/930/3e2321b0-d54a-46c2-bb84-007bb57eb381/640/3e2321b0-d54a-46c2-bb84-007bb57eb381.png)
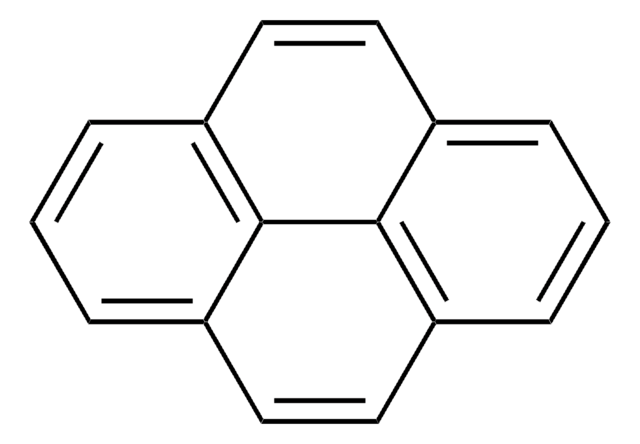
![Benz[a]anthracene 99%](/deepweb/assets/sigmaaldrich/product/structures/351/486/b3ddf157-a732-4ef8-83f0-c70a53404cb2/640/b3ddf157-a732-4ef8-83f0-c70a53404cb2.png)