추천 제품
Grade
for catalysis
Quality Level
분석
≥98.5% (HPLC)
불순물
≤0.05% water
refractive index
n20/D 1.52
density
1.21 g/mL at 20 °C (lit.)
음이온 미량물
bromide (Br-): ≤25 mg/kg
chloride (Cl-): ≤25 mg/kg
nitrate (NO3-): ≤25 mg/kg
phosphate (PO43-): ≤25 mg/kg
sulfate (SO42-): ≤10 mg/kg
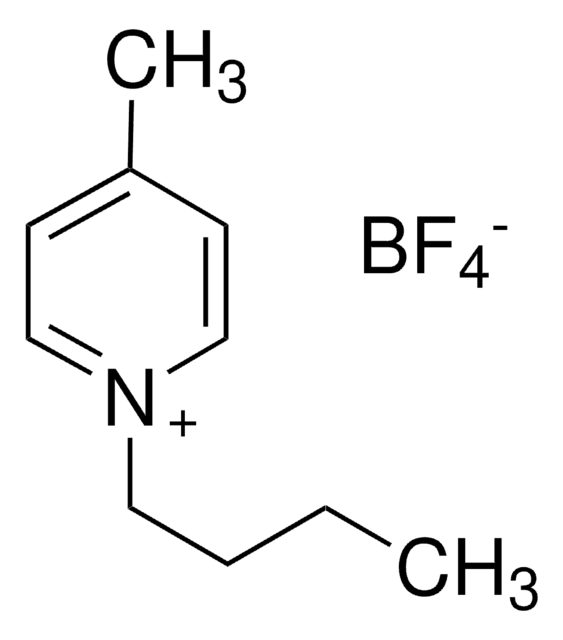
SMILES string
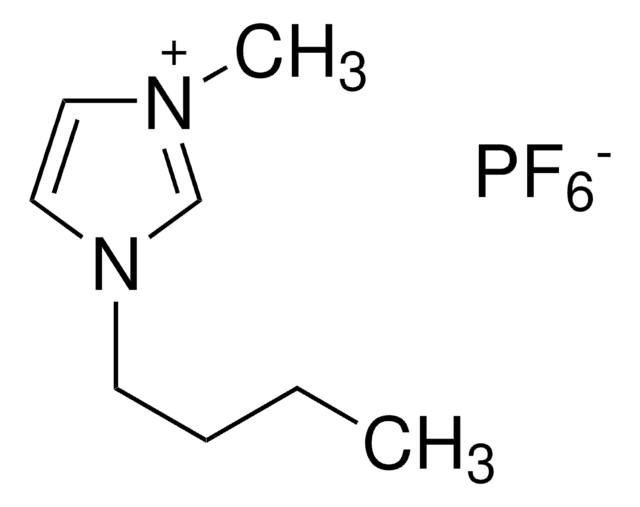
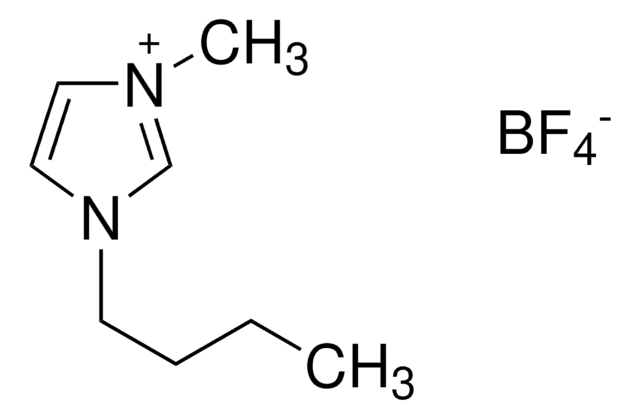
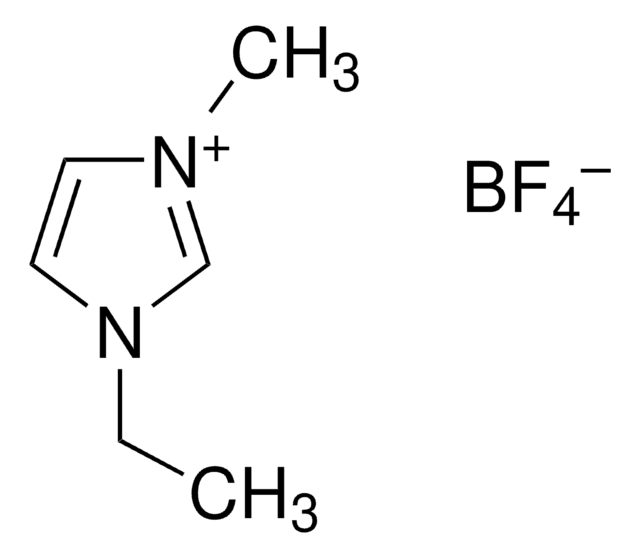
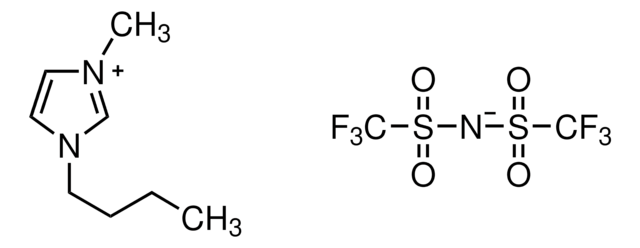
F[B-](F)(F)F.CCCCn1cc[n+](C)c1
InChI
1S/C8H15N2.BF4/c1-3-4-5-10-7-6-9(2)8-10;2-1(3,4)5/h6-8H,3-5H2,1-2H3;/q+1;-1
InChI key
LSBXQLQATZTAPE-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
애플리케이션
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 3 Oral - Aquatic Chronic 2 - Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point (°F)
550.4 °F
Flash Point (°C)
288 °C
개인 보호 장비
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
이미 열람한 고객
문서
Ionic Liquids have been thoroughly investigated as solvents in most types of catalytic reactions. Their merit lies in the ease with which their physical–chemical properties can be tuned by varying either the anion, the cation, or its substitution pattern.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.