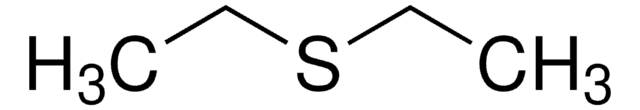추천 제품
Grade
anhydrous
Quality Level
vapor density
2.1 (vs air)
vapor pressure
26.24 psi ( 55 °C)
7.79 psi ( 20 °C)
분석
≥99.0%
형태
liquid
autoignition temp.
402 °F
expl. lim.
19.7 %
불순물
<0.003% water
<0.005% water (100 mL pkg)
증발 잔류물
<0.0005%
refractive index
n20/D 1.435 (lit.)
bp
38 °C (lit.)
mp
−98 °C (lit.)
solubility
H2O: soluble 7.28 g/L at 20 °C
density
0.846 g/mL at 25 °C (lit.)
SMILES string
CSC
InChI
1S/C2H6S/c1-3-2/h1-2H3
InChI key
QMMFVYPAHWMCMS-UHFFFAOYSA-N
유전자 정보
rat ... Maoa(29253) , Maob(25750)
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Dimethyl sulfide is the major biogenic, volatile sulfur compound that is released from the ocean to the atmosphere. It plays a main role in the global sulfur cycle.
애플리케이션
Dimethyl sulfide undergoes thiolation reaction with hydrogen sulfide (H2S) in the presence of tungsten-zirconia (WO3/ZrO2) catalyst to form methanethiol.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point (°F)
-32.8 °F - closed cup
Flash Point (°C)
-36 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves
Choose from one of the most recent versions:
이미 열람한 고객
Photooxidation of dimethyl sulfide and dimethyl disulfide. I: Mechanism development
Yin F, et al
Journal of Atmospheric Chemistry, 11(4), 309-364 (1990)
Ingrid Elisia et al.
PloS one, 11(3), e0152538-e0152538 (2016-04-01)
Dimethyl sulfoxide (DMSO) is currently used as an alternative treatment for various inflammatory conditions as well as for cancer. Despite its widespread use, there is a paucity of data regarding its safety and efficacy as well as its mechanism of
Thiolation of dimethyl sulfide to methanethiol over WO3/ZrO2 catalysts
Chen S, et al
J. Mol. Catal. A: Chem., 365, 60-65 (2012)
Thiolation of dimethyl sulfide to methanethiol over WO3/ZrO2 catalysts.
Chen S, et al.
J. Mol. Catal. A: Chem., 365, 60-65 (2012)
Dimethyl sulfide production during natural phytoplanktonic blooms.
Nguyen BC, et al.
Marine Chemistry, 24(2), 133-141 (1988)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.