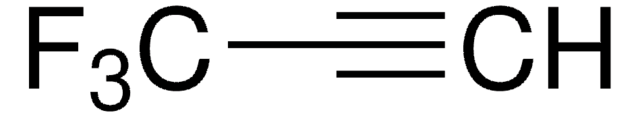모든 사진(1)
About This Item
Linear Formula:
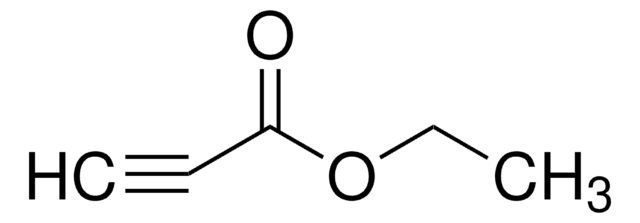
CF3C≡CCO2C2H5
CAS Number:
Molecular Weight:
166.10
Beilstein:
3539414
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
일반 설명
Ethyl 4,4,4-trifluoro-2-butynoate is an unsymmetrical internal alkyne.
애플리케이션
Ethyl 4,4,4-trifluoro-2-butynoate has been used to investigate the regioselectivity of the insertion reaction with cyclometalated iridium and rhodium complexes.
It may be used in the synthesis of the following compounds :
It may be used in the synthesis of the following compounds :
- (2S,4aR,6aR,7R,9S,10aS,10bR)-methyl 9-acetoxy-2-(5-(ethoxycarbonyl)-6-(trifluoromethyl)-7-oxabicyclo[2.2.1]hepta-2,5-dien-2-yl)-6a,10b-dimethyl-4,10-dioxododecahydro-1H-benzo[f]isochromene-7-carboxylate
- (2S,4aR,6aR,7R,9S,10aS,10bR)-methyl 9-acetoxy-2-(6-(ethoxycarbonyl)-5-(trifluoromethyl)-7-oxabicyclo[2.2.1]hepta-2,5-dien-2-yl)-6a,10b-dimethyl-4,10-dioxododecahydro-1Hbenzo[f]isochromene-7-carboxylate
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point (°F)
42.8 °F - closed cup
Flash Point (°C)
6 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
Reactivity and Regioselectivity of Insertion of Unsaturated Molecules into M- C (M= Ir, Rh) Bonds of Cyclometalated Complexes.
Li L, et al.
Organometallics, 29(20), 4593-4605 (2010)
Anthony Lozama et al.
Journal of natural products, 74(4), 718-726 (2011-02-23)
As part of our continuing efforts toward more fully understanding the structure-activity relationships of the neoclerodane diterpene salvinorin A, we report the synthesis and biological characterization of unique cycloadducts through [4+2] Diels-Alder cycloaddition. Microwave-assisted methods were developed and successfully employed
Facile access to stereodefined dienoates and cyclopropylenoates containing a trifluoromethyl group.
Wang P-A, et al.
Journal of Fluorine Chemistry, 124(1), 93-97 (2003)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.