추천 제품
분석
98%
형태
solid
mp
287-289 °C (lit.)
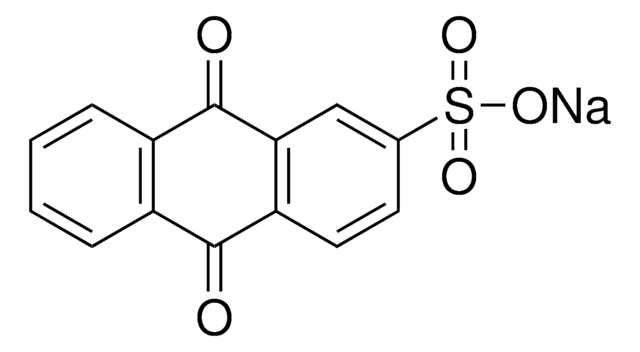
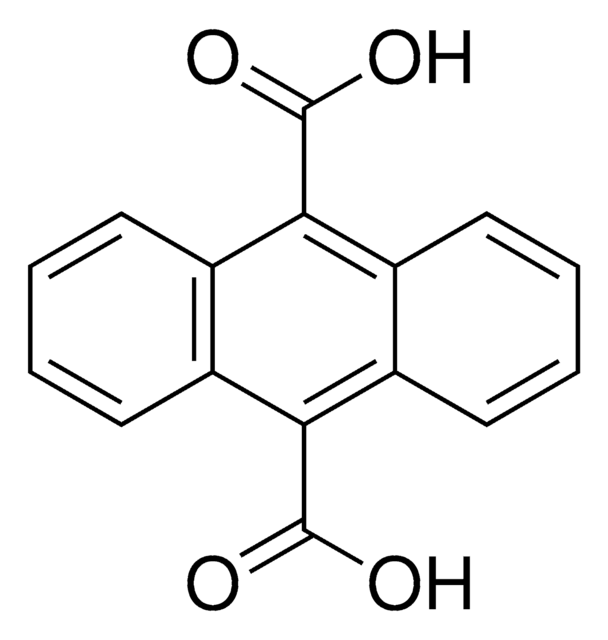
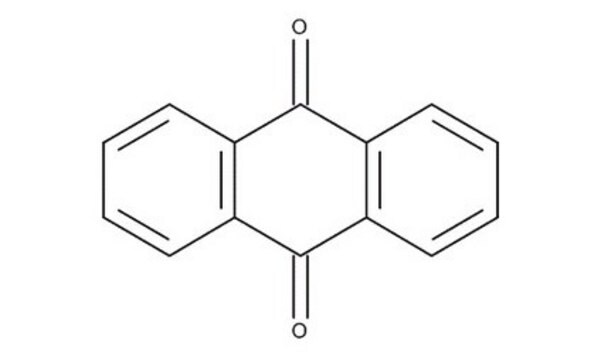
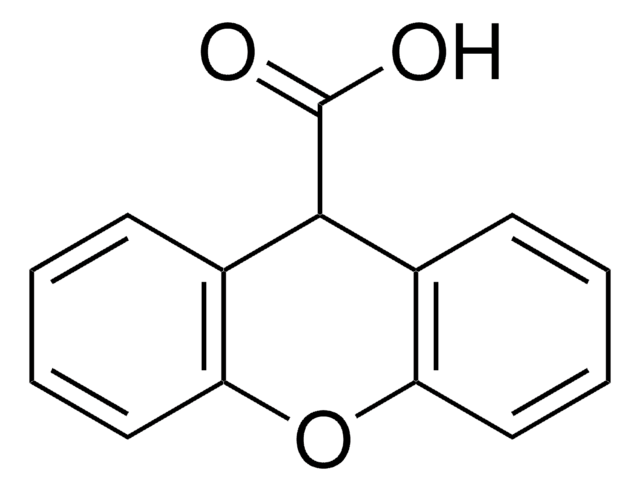
SMILES string
OC(=O)c1ccc2C(=O)c3ccccc3C(=O)c2c1
InChI
1S/C15H8O4/c16-13-9-3-1-2-4-10(9)14(17)12-7-8(15(18)19)5-6-11(12)13/h1-7H,(H,18,19)
InChI key
ASDLSKCKYGVMAI-UHFFFAOYSA-N
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
이미 열람한 고객
S Y Tsai et al.
Journal of pharmaceutical sciences, 82(12), 1250-1254 (1993-12-01)
The physicochemical properties of 9,10-anthraquinone-2-carboxylic acid (AQCA) were investigated by thermal analysis, powder X-ray diffraction pattern, solubility, and partition coefficient. The chemical structure of AQCA was confirmed by the data from UV, Fourier transform IR (FTIR), and NMR analyses. Solubility
Coupled Proton and Electron Transfer: Adsorbed Anthraquinone-2-carboxylic Acid Monolayers.
Forster RJ.
Journal of the Electrochemical Society, 144(4), 1165-1173 (1997)
Yiwen Zhu et al.
Materials (Basel, Switzerland), 13(16) (2020-08-17)
Antimicrobial and antiviral materials have attracted significant interest in recent years due to increasing occurrences of nosocomial infections and pathogenic microbial contamination. One method to address this is the combination of photoactive compounds that can produce reactive oxygen species (ROS)
A Bielawska et al.
Folia histochemica et cytobiologica, 39 Suppl 2, 207-208 (2002-02-01)
Although prolidase [E.C.3.4.13.9] is found in normal cells, substantially increased levels are found in some neoplastic tissues. Prolidase evokes the ability to hydrolyse the imido-bond of various low molecular weight compounds coupled to L-proline. The synthesis of three proline analogues
A Bielawska et al.
Roczniki Akademii Medycznej w Bialymstoku (1995), 43, 201-209 (1999-02-11)
The feasibility to targeting prolidase as an antineoplastic prodrug--converting enzyme has been examined. The synthesis of proline analogue of anthraquinone-2-carboxylic acid (potential antineoplastic agent) conjugated through imido-bond (potential target for prolidase action) has been performed. The product was found to
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.