추천 제품
vapor pressure
0.01 mmHg ( 25 °C)
분석
≥99%
형태
solid
bp
260-262 °C (lit.)
mp
39-41 °C (lit.)
solubility
alcohol: freely soluble(lit.)
benzene: freely soluble(lit.)
chloroform: freely soluble(lit.)
diethyl ether: freely soluble(lit.)
petroleum ether: very slightly soluble(lit.)
water: very slightly soluble(lit.)
SMILES string
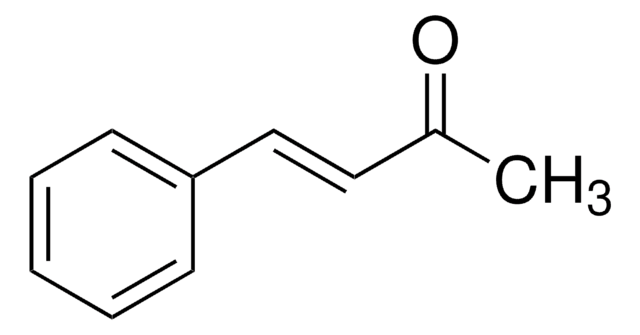
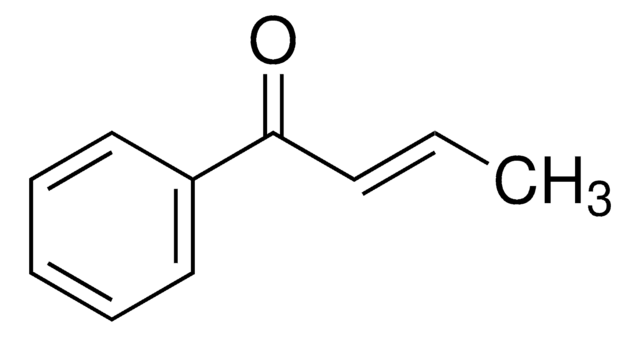
CC(=O)\C=C\c1ccccc1
InChI
1S/C10H10O/c1-9(11)7-8-10-5-3-2-4-6-10/h2-8H,1H3/b8-7+
InChI key
BWHOZHOGCMHOBV-BQYQJAHWSA-N
관련 카테고리
일반 설명
trans-4-Phenyl-3-buten-2-one forms trans-4-(4-hydroxyphenyl)-3-buten-2-one (4-OH-PBO) as a metabolite when incubated with liver microsomes of untreated rats in the presence of NADPH.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - Skin Sens. 1 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
>230.0 °F
Flash Point (°C)
> 110 °C
개인 보호 장비
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
Hiroyuki Morita et al.
Proceedings of the National Academy of Sciences of the United States of America, 107(2), 669-673 (2010-01-19)
Benzalacetone synthase (BAS), a plant-specific type III polyketide synthase (PKS), catalyzes a one-step decarboxylative condensation of malonyl-CoA and 4-coumaroyl-CoA to produce the diketide benzalacetone. We solved the crystal structures of both the wild-type and chalcone-producing I207L/L208F mutant of Rheum palmatum
Ikuro Abe et al.
The Journal of biological chemistry, 278(27), 25218-25226 (2003-05-02)
Benzalacetone synthase (BAS) and chalcone synthase (CHS) are plant-specific type III polyketide synthases (PKSs) that share approximately 70% amino acid sequence identity. BAS catalyzes a one-step decarboxylative condensation of 4-coumaroyl-CoA with malonyl-CoA to produce a diketide benzalacetone, whereas CHS performs
Samyeol Seo et al.
Applied and environmental microbiology, 78(11), 3816-3823 (2012-03-27)
The entomopathogenic bacteria Xenorhabdus nematophila and Photorhabdus temperata subsp. temperata suppress insect immune responses by inhibiting the catalytic activity of phospholipase A(2) (PLA(2)), which results in preventing biosynthesis of immune-mediating eicosanoids. This study identified PLA(2) inhibitors derived from culture broths
Lisa Dalla Via et al.
European journal of medicinal chemistry, 44(7), 2854-2861 (2009-01-22)
A series of conjugates of alpha,beta-unsaturated ketone systems, phenyl-butenone and diaryl-propenones (chalcones), with the tricyclic planar pyrroloquinoline nucleus were synthesised and evaluated for their anticancer properties. The aim was to target DNA by butenone and chalcones, and determine the occurrence
Toshiyuki Wakimoto et al.
Methods in enzymology, 515, 337-358 (2012-09-25)
Members of the chalcone synthase superfamily of type III polyketide synthases (PKSs) catalyze iterative condensations of CoA thioesters to produce a variety of polyketide scaffolds with remarkable structural diversity and biological activities. The homodimeric type III PKSs share a common
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.