Cholesterol Oxidase Assay Procedure
Principle

The appearance of quinoneimine dye formed when coupled with 4-aminoantipyrine and phenol is measured at 500nm by spectrophotometry.
Unit definition
One unit causes the formation of one micromole of hydrogen peroxide (half a micromole of quinoneimine dye) per minute under the conditions described below.
Method
Reagents
Procedure
- Prepare the following working solution (20 tests volume), immediately before use and store on ice in a brownish bottle.
51.0mL Buffer solution (A)
4.0mL Substrate solution (B)
1.0mL 4-AA solution (C)
2.0mL POD solution (E)
- Pipette 2.9mL of working solution into a cuvette (d=1.0cm) and equilibrate at 37 ℃ for about 3 minutes. Add 0.1mL of Phenol solution (D), mix and keep at 37 ℃ for another 2 minutes.
- Add 0.1mL of the enzyme solution* and mix with gentle inversion.
- Record the increase in optical density at 500nm against water for 3 to 4 minutes in a spectrophotometer thermostated at 37 ℃, and calculate the ΔOD per minute from the linear portion of the curve (ΔOD test).
* At the same time, measure the blank rate (ΔOD blank) by using the same method as the test except that the enzyme diluent is added instead of the enzyme solution. Dissolve the enzyme preparation in ice-cold enzyme diluent (F), and dilute to 0.1-0.3U/mL with the same buffer, and store on ice.
Calculation
Activity can be calculated by using the following formula:
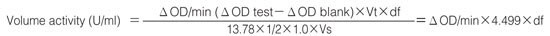
Weight activity (U/mg)=(U/mL)×1/C
This procedure is for informational purposes. For a current copy of our quality control procedure contact our Technical Service Department.
To continue reading please sign in or create an account.
Don't Have An Account?