71010-576BC
GDS Listeria monocytogenes Tq
BioControl, Molecular-based PCR assay suitable for detection of Listeria monocytogenes in food and environmental samples.
Synonym(s):
Nucleic acid amplification system
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
UNSPSC Code:
41106302
Recommended Products
Agency
according to Immunomagnetic Separation (IMS)
meets requirements for AOAC® PTM
meets requirements for Health Canada
meets requirements for MicroVal
Quality Level
shelf life
6-12 mo.
feature
0.1 mL amp tubes
packaging
pkg of 576 tests
manufacturer/tradename
Assurance GDS®
technique(s)
RT-PCR: suitable
application(s)
food and beverages
compatibility
configured for BioControl Systems GDS Rotor-Gene
configured for BioControl Systems GDS
storage temp.
2-8°C
suitability
Listeria monocytogenes
General description
Assurance GDS®, genetic detection system, for Listeria monocytogenes Tq is an automated nucleic acid amplification system for the detection of Listeria monocytogeness in food and environmental samples. The proprietary PickPen® IMS-based sample preparation procedure quickly and effectively captures Listeria monocytogenes, separating the pathogen from inhibitory background materials ensuring valid PCR reactions for all sample types, including challenging food and environmental samples. Assurance GDS® PCR technology provides two additional levels of specificity through the use of both highly specific primers and probes to ensure accurate detection of Listeria monocytogenes.
Application
GDS, genetic detection system, for Listeria monocytogenes Tq is an automated nucleic acid amplification system for the detection of Listeria monocytogenes in foods, ingredients, and environmental samples.
Features and Benefits
- Results in 24 hours for environmental samples and select foods
- Single-step enrichment reduces handling time
- Validated for liquid milk, Mexican soft cheese, frankfurters, deli turkey, raw fish, raw green beans, stainless steel, rubber, concrete, plastic, among others
- Features innovative PickPen® immunomagnetic separation (IMS) for additional specificity and faster time to results
- Simplified sample preparation does not require heating blocks or preparation of reagents
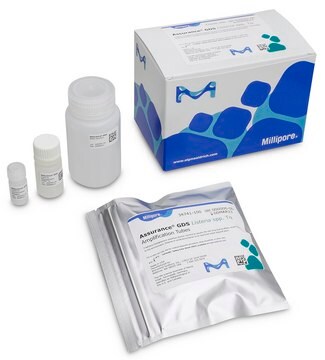
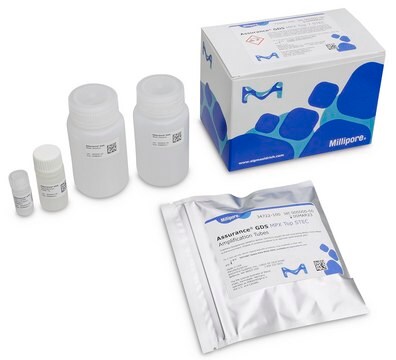
Components
Each 576 test kit includes:
NOTE: The 100 kit includes standard 0.2 mL amp tubes whereas the 576 kit includes smaller 0.1 mL amp tubes. The 576 kit requires additional equipment tools to process per the kit Directions for Use.
- Listeria monocytogenes Amplification Tubes Tq (0.1 mL)
- Listeria Concentration Reagent
- Listeria Resuspension Buffer Tq
- Wash Solution
NOTE: The 100 kit includes standard 0.2 mL amp tubes whereas the 576 kit includes smaller 0.1 mL amp tubes. The 576 kit requires additional equipment tools to process per the kit Directions for Use.
Compatibility
BioControl® GDS
Testing Method
PCR, Immunomagnetic Separation (IMS)
Legal Information
AOAC is a registered trademark of AOAC International
Assurance is a registered trademark of Merck KGaA, Darmstadt, Germany
Assurance GDS is a registered trademark of BioControl Systems, Inc.
BioControl is a registered trademark of BioControl Systems, Inc.
PICKPEN is a registered trademark of Merck KGaA, Darmstadt, Germany
configured for
Product No.
Description
Pricing
required but not provided
Product No.
Description
Pricing
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service