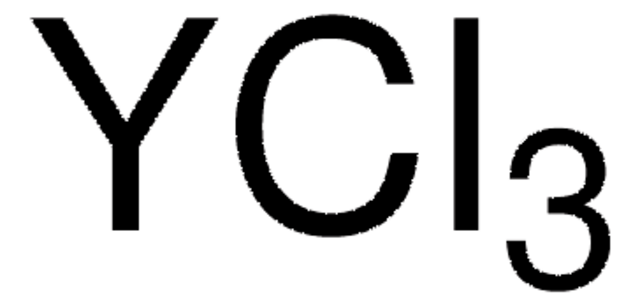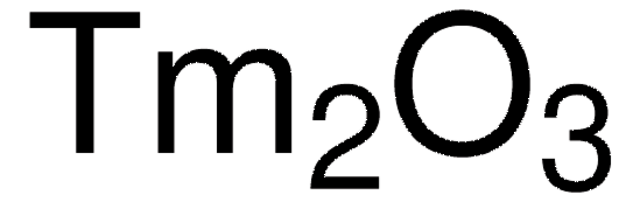439649
Thulium(III) chloride
anhydrous, powder, 99.9% trace metals basis
Synonym(s):
Thulium trichloride
About This Item
Recommended Products
grade
anhydrous
Assay
99.9% trace metals basis
form
powder
reaction suitability
core: thulium
reagent type: catalyst
SMILES string
Cl[Tm](Cl)Cl
InChI
1S/3ClH.Tm/h3*1H;/q;;;+3/p-3
InChI key
ILOTUXNTERMOJL-UHFFFAOYSA-K
Related Categories
General description
Application
accessory
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
The rare earth elements impact nearly everyone in the world. All of the people living in advanced technological countries and almost all those living in third world countries utilize the rare earths in their everyday living—the car that one drives (gasoline is refined from oil using rare earth catalysts and catalytic converters reduce the polluting emissions from the automotive exhaust), watching the news on TV (the red and green colors in TV screens), the telephones and computers we use to communicate (the permanent magnets in speakers and disc drives), just to name a few examples.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service