T0303
Trypsin from porcine pancreas
Type IX-S, lyophilized powder, 13,000-20,000 BAEE units/mg protein
Synonym(s):
Serine Protease 1
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Recommended Products
type
Type IX-S
Quality Level
form
lyophilized powder
specific activity
13,000-20,000 BAEE units/mg protein
mol wt
23.8 kDa
application(s)
diagnostic assay manufacturing
foreign activity
chymotrypsin ≤1.0 units/mg protein
storage temp.
−20°C
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
For trypsin digestion of peptides, use a ratio of about 1:100 to 1:20 for trypsin:peptide. The typical use for this product is in removing adherent cells from a culture surface. The concentration of trypsin necessary to dislodge cells from their substrate is dependent primarily on the cell type and the age of the culture. Trypsins have also been used for the re-suspension of cells during cell culture, in proteomics research for digestion of proteins and in various in-gel digestionsns†. Additional applications include assessing crystallization by membrane-based techniques and in a study to determine that protein folding rates and yields can be limited by the presence of kinetic traps.
Biochem/physiol Actions
Trypsin cleaves peptides on the C-terminal side of lysine and arginine residues. The rate of hydrolysis of this reaction is slowed if an acidic residue is on either side of the cleavage site and hydrolysis is stopped if a proline residue is on the carboxyl side of the cleavage site. The optimal pH for trypsin activity is 7-9. Trypsin can also act to cleave ester and amide linkages of synthetic derivatives of amino acids. EDTA is added to trypsin solutions as a chelating agent that neutralizes calcium and magnesium ions that obscure the peptide bonds on which trypsin acts. Removing these ions increases the enzymatic activity.
Serine protease inhibitors, including DFP, TLCK, APMSF, AEBSEF, and aprotinin, amongst others, will inhibit Trypsin.
Serine protease inhibitors, including DFP, TLCK, APMSF, AEBSEF, and aprotinin, amongst others, will inhibit Trypsin.
Components
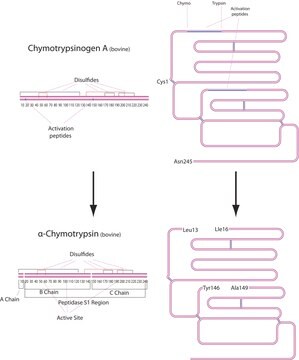
Trypsin consists of a single chain polypeptide of 223 amino acid residues, produced by the removal of the N-terminal hexapeptide from trypsinogen which is cleaved at the Lys - lle peptide bond. The sequence of amino acids is cross-linked by 6 disulfide bridges. This is the native form of trypsin, beta-trypsin. BETA-trypsin can be autolyzed, cleaving at the Lys - Ser residue, to produce alpha-trypsin. Trypsin is a member of the serine protease family.
Caution
Solutions in 1 mM HCl are stable for 1 year in aliquots and stored at -20°C. The presence of Ca2+ will also diminish the self-autolysis of trypsin and maintain its stability in solution. Trypsin will also retain most of its activity in 2.0 M urea, 2.0 M guanidine HCl, or 0.1% (w/v) SDS.
Unit Definition
One BAEE unit will produce a ΔA253 of 0.001 per min at pH 7.6 at 25° C using BAEE as substrate. One BTEE unit = 320 ATEE units. Reaction volume = 3.2 mL (1 cm light path).
Preparation Note
This product is a lyophilized powder soluble in Hank′s Balanced Salt Solution at 25 mg/mL.
inhibitor
Product No.
Description
Pricing
substrate
Product No.
Description
Pricing
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Resp. Sens. 1 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
G K German et al.
Biophysical journal, 102(11), 2424-2432 (2012-06-21)
We study the drying of stratum corneum, the skin's outermost layer and an essential barrier to mechanical and chemical stresses from the environment. Even though stratum corneum exhibits structural features across multiple length-scales, contemporary understanding of the mechanical properties of
S Rajeshkumar et al.
Fish & shellfish immunology, 26(3), 429-437 (2009-01-15)
The protective efficacy of oral delivery of a DNA construct containing the VP28 gene of WSSV encapsulated in chitosan nanoparticles was investigated in black tiger shrimp (Penaeus monodon). The results showed that significant survival was obtained in WSSV-challenged shrimp at
Sulena Polez et al.
PloS one, 11(12), e0167207-e0167207 (2016-12-03)
A significant barrier to insulin is affordability. In this manuscript we describe improvements to key steps in the insulin production process in Pichia pastoris that reduce cost and time. The strategy for recovery and processing of human insulin precursor has
Jelle Matthijnssens et al.
Journal of virology, 84(4), 2013-2026 (2009-11-27)
Although few simian rotaviruses (RVs) have been isolated, such strains have been important for basic research and vaccine development. To explore the origins of simian RVs, the complete genome sequences of strains PTRV (G8P[1]), RRV (G3P[3]), and TUCH (G3P[24]) were
Karol Szczepanek et al.
The Journal of biological chemistry, 286(34), 29610-29620 (2011-07-01)
Expression of the STAT3 transcription factor in the heart is cardioprotective and decreases the levels of reactive oxygen species. Recent studies indicate that a pool of STAT3 resides in the mitochondria where it is necessary for the maximal activity of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service