Metathesis
Olefin methathesis is an organic reaction that features the rearrangement of carbon-carbon double bonds by the redistribution of alkene fragments. It has led scientists to discover new disconnections in organic synthesis, paving the way for new advances in polymer chemistry, drug discovery, and natural product synthesis. There are three main classes of metathesis reactions, ring-closing metathesis and cross metathesis are utilized regularly for small molecule organic synthesis while ring-opening metathesis is frequently used for polymerization.
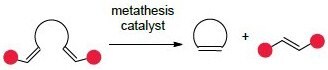
The most common and recognizable application of olefin metathesis in organic synthesis is ring-closing metathesis (RCM), an intramolecular reaction of an acyclic diene to form a ring. This methodology allows for the construction of all-carbon and heteroatom-containing rings that are rich in sp3-centers, a growing theme in modern medicinal chemistry.1
Cross metathesis brings two olefins together in an intermolecular reaction to give an olefin product bearing substituent from each of the starting olefins. Excellent functional group compatibility, in addition to a tolerance of residual moisture and oxygen, have facilitated the broad acceptance of ruthenium-catalyzed macrocyclization as a general methodology for the preparation of large rings (≥12 atoms).2
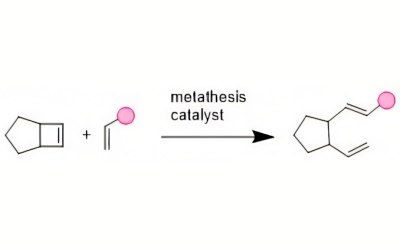
Ring-opening metathesis polymerization (ROMP) designs polymers with tunable properties and has become a powerful technological advancement in the arena of facilitated synthesis. Due to the low cost and readily available starting materials, this robust polymerization methodology can be used to produce polymers on a large scale.3
Related Technical Articles
- Metal-enhanced organic synthesis via organometallic intermediates is a widely used preparative route for thousands of organic compounds.
- Lipshutz and co-workers have recently developed a second generation technology to their original PTS-enabling surfactant based on the polyoxyethanyl-α-tocopheryl succinate derivative, TPGS-750-M.
- This robust protocol for hydrosilylation of terminal acetylenes to give α-vinylsilanes using [Cp*Ru(MeCN)3]PF6, as well as a number of other catalysts for hydrosilylation, comes from the Trost group at Stanford University.
- Olefin metathesis is now a well-entrenched synthetic technique, and is a powerful method for the clean construction of innumerable classes of chemical architectures.
- Rovis has demonstrated that triazolium salt in the presence of a base can act as an N-heterocyclic carbene organocatalyst1 in highly enantioselective intramolecular Stetter reactions.
- See All (16)
References
To continue reading please sign in or create an account.
Don't Have An Account?