おすすめの製品
品質水準
アッセイ
≥98% (HPLC)
形状
powder
色
white to beige
溶解性
DMSO: ≥15 mg/mL
保管温度
2-8°C
SMILES記法
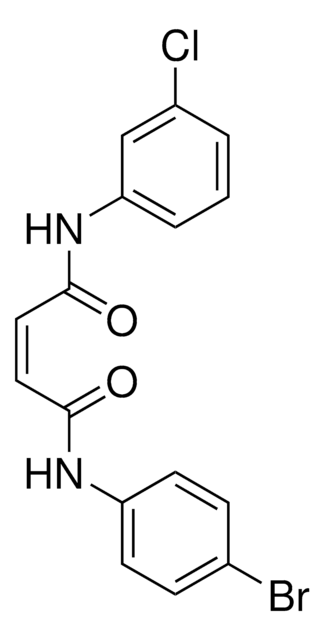
Cc1ccc(NC(=O)CCCCCC(=O)Nc2ccccc2N)cc1
InChI
1S/C20H25N3O2/c1-15-11-13-16(14-12-15)22-19(24)9-3-2-4-10-20(25)23-18-8-6-5-7-17(18)21/h5-8,11-14H,2-4,9-10,21H2,1H3,(H,22,24)(H,23,25)
InChI Key
WTKBRPXPNAKVEQ-UHFFFAOYSA-N
生物化学的/生理学的作用
106 is a Class I HDAC inhibitor, demonstrating no activity against class II HDACs. It is a slow, tight-binding inhibitor of HDACs 1, 2, and 3, with a preference toward HDAC3 with Ki of 14 nM, 15 times lower than the Ki for HDAC1.
Pimelic diphenylamides has the ability to enhance the expression of the frataxin gene in lymphocytes from Friedreich ataxia patients.
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
試験成績書(COA)
製品のロット番号・バッチ番号を入力して、試験成績書(COA) を検索できます。ロット番号・バッチ番号は、製品ラベルに「Lot」または「Batch」に続いて記載されています。
Pimelic diphenylamide 106 is a slow, tight-binding inhibitor of class I histone deacetylases
Chou CJ, et al.
The Journal of Biological Chemistry, 283(51), 35402-35409 (2008)
Yifei Liao et al.
PLoS pathogens, 17(2), e1009307-e1009307 (2021-02-18)
Marek's disease virus (MDV) is a potent oncogenic alphaherpesvirus that elicits a rapid onset of malignant T-cell lymphomas in chickens. Three MDV types, including GaHV-2 (MDV-1), GaHV-3 (MDV-2) and MeHV-1 (HVT), have been identified and all encode a US3 protein
Jason A Pfister et al.
PloS one, 14(4), e0215208-e0215208 (2019-04-12)
SIRT1, a NAD+-dependent deacetylase, protects neurons in a variety of in vitro and in vivo models of neurodegenerative disease. We have previously described a neuroprotective effect by SIRT1 independent of its catalytic activity. To confirm this conclusion we tested a
Dominik Stammler et al.
Journal of immunology (Baltimore, Md. : 1950), 195(11), 5421-5431 (2015-11-01)
Histone deacetylase (HDAC) inhibitors (HDACi) are clinically approved anticancer drugs that have important immune-modulatory properties. We report the surprising finding that HDACi promote LPS-induced IL-1β processing and secretion in human and murine dendritic cells and murine macrophages. HDACi/LPS-induced IL-1β maturation
Angela K Carrillo et al.
Bioorganic & medicinal chemistry, 23(16), 5151-5155 (2015-02-01)
Two of the histone deacetylases, TbDAC1 and TbDAC3, have been reported to be essential genes in trypanosomes. Therefore, we tested the activity of a panel of human histone deacetylase inhibitors (HDACi) for their ability to block proliferation of Trypanosoma brucei
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)