おすすめの製品
品質水準
アッセイ
≥99.0% (NT)
形状
powder
光学活性
[α]20/D +15.5±1°, c = 1% in 1 M HCl
反応適合性
reaction type: solution phase peptide synthesis
mp
259 °C (dec.) (lit.)
アプリケーション
peptide synthesis
SMILES記法
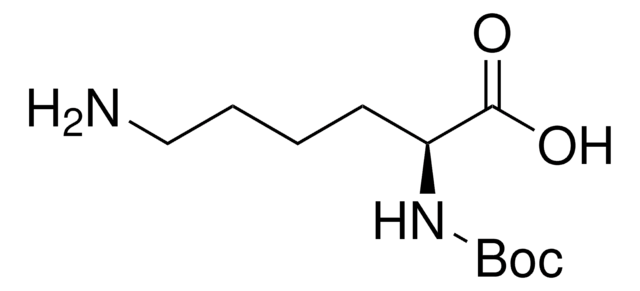
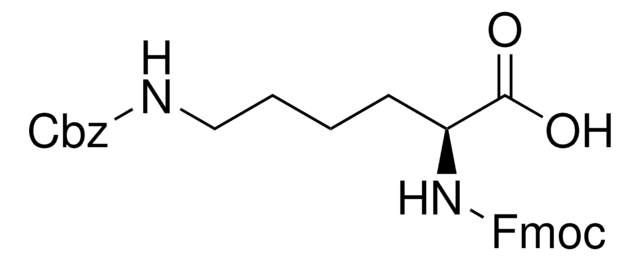
N[C@@H](CCCCNC(=O)OCc1ccccc1)C(O)=O
InChI
1S/C14H20N2O4/c15-12(13(17)18)8-4-5-9-16-14(19)20-10-11-6-2-1-3-7-11/h1-3,6-7,12H,4-5,8-10,15H2,(H,16,19)(H,17,18)/t12-/m0/s1
InChI Key
CKGCFBNYQJDIGS-LBPRGKRZSA-N
類似した製品をお探しですか? 訪問 製品比較ガイド
関連するカテゴリー
詳細
H-Lys(Z)-OH also known as N6-carbobenzyloxy-L-lysine, commonly used as a reagent in the synthesis of peptides.
アプリケーション
H-Lys(Z)-OH serves as a reactant for the synthesis of various peptides such as boc-Glu(OBzl)-Lys(Z)-OH and tert-butyl-6-(((benzyloxy)carbonyl)amino)-2-bromohexanoate. Additionally, it is used in the Fuchs-Farthing method to synthesize lysine NCA, which is a block copolymer.
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
個人用保護具 (PPE)
Eyeshields, Gloves, type N95 (US)
試験成績書(COA)
製品のロット番号・バッチ番号を入力して、試験成績書(COA) を検索できます。ロット番号・バッチ番号は、製品ラベルに「Lot」または「Batch」に続いて記載されています。
この製品を見ている人はこちらもチェック
R L Hanson et al.
Applied microbiology and biotechnology, 37(5), 599-603 (1992-08-01)
Biotransformations were developed to oxidize N epsilon-carbobenzoxy(CBZ)-L-lysine and to reduce the product keto acid to L-CBZ-oxylysine. Lysyl oxidase (L-lysine: O2 oxidoreductase, EC 1.4.3.14) from Trichoderma viride was relatively specific for L-lysine and had very low activity with N epsilon-substituted derivatives.
J Zheng et al.
Biotechnology progress, 16(2), 254-257 (2000-04-08)
Poly(epsilon-CBZ-L-lysine) can be mixed with biodegradable polymers such as poly(D,L-lactic-co-glycolic acid) or poly(L-lactic acid) and formed into films, foams, or microspheres. Surface amino groups may then be deprotected with acid or lithium/liquid ammonia. The amino groups serve as a method
J Zheng et al.
Biotechnology progress, 15(4), 763-767 (1999-08-12)
Microspheres were formed from blends of the biodegradable polymer poly(DL-lactic-co-glycolic acid) (PLGA) together with poly(epsilon-CBZ-L-lysine) (PCBZL) by a double-emulsification/solvent evaporation technique. The size of the microspheres formed by this method was dependent both on the total concentration of the polymers
Christopher D Reinkemeier et al.
Cell, 184(19), 4886-4903 (2021-08-26)
Engineering new functionality into living eukaryotic systems by enzyme evolution or de novo protein design is a formidable challenge. Cells do not rely exclusively on DNA-based evolution to generate new functionality but often utilize membrane encapsulation or formation of membraneless
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)