Cell Culture Troubleshooting


Cell culture has become one of the most fundamental techniques for modeling biological systems, and is of increasing importance in the biotechnology and pharmaceutical sectors as well as an essential process in life science research labs. Though this technique is highly accessible, successful propagation of cells for stock expansion or modeling experiments can be plagued by contamination or other conditions that negatively impact cell viability. The common practice of sharing cells has led to well-published evidence of cross-contamination of cell stocks whose identity was previously unquestioned. Identifying both common and infrequent reasons why cells may fail to thrive can increase lab efficiency, enhance yield of cellular products, and ensure meaningful, reliable downstream data from in vitro models.

Cell Line Misidentification
Recent studies estimate misidentification may affect up to one third of all cell lines in use. These discoveries have the potential to call into question published findings about biological systems based on results from cell line models. Factors leading to cell line misidentification/cross-contamination include:
- Contamination with aggressive cell lines: Isoenzyme analysis has demonstrated that many cell lines share a rare enzyme isoform with HeLa cells. HeLa has since been shown to be the most common invasive cell line in culture stocks.
- Documentation and labeling practices: Cell culture flasks, plates, cryovials, and freezer containment must be clearly labeled, and liquid nitrogen storage maps strictly maintained to reflect the addition, withdrawal, and relocation of cell stocks.
- Cell line borrowing: Sharing of cells amongst neighboring labs is a common practice that leads to errors in cell line identity. Cell lines should only be obtained from reputable cell repositories such as ECACC, the European Collection of Authenticated Cell Cultures at Public Health England.
Be informed about common causes of cell line cross-contamination and how to protect your work from its consequences.

Microbial Contamination of Cell Cultures
Cell culture media and incubation conditions provide the ideal environment for cells, as well as bacterial, fungal, and viral contaminants. To augment diligent adherence to aseptic culture technique, cultures must be regularly examined microscopically for evidence of bacterial and fungal intruders. Some ubiquitous microbial contaminants evade visual detection, as up to 30% of all cultures are estimated to be contaminated with mycoplasma. Reagents and kits designed for detection of invisible mycoplasma are often based on PCR amplification, which can also be used to detect viral contaminants.
Learn how to keep your cultures free of biological and chemical contaminants.

Poor Cell Growth
It's frustrating when cells in culture that otherwise appear healthy fail to attain confluency. When contaminants are ruled out, some possible barriers to healthy cell doubling include:
- Culture/freezing media condition and quality
- Quality and application-suitability of supplements
- Inaccurate cell enumeration during freezedown or passage
When cells seem viable but fail to expand, learn what you can do to improve culture conditions for optimal growth before heading back to the liquid nitrogen.

Detachment of Adherent Cell Phenotypes
Adherent cells may spontaneously detach in culture, singly or in sheets. When adherent cultures don't stay that way, know what to check and how to restore cohesion in culture.
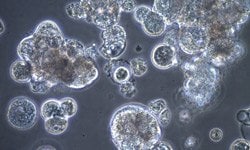
Cell Clumping
Cells in suspension mimic in vivo conditions as singlets. When cells start to clump, this can be due to sticky nucleic acids, present in media when cultures are stressed. What's hiding in media that can cause cells to clump? Read more here about how to banish clumping from cultures.

Cell Death in Culture
When cells die in culture, contamination with microorganisms must first be ruled out. Other factors that may contribute to poor cell health or behavior inconsistent with phenotype include:
- Frozen stock conditions
- Cell passage number/overconfluency
- Environmental stress
- Overdigestion with dissociation enzymes
Appropriate equipment, qualified reagents, and expert protocols are key to resolving unexpected cell culture outcomes.

Precipitates in Culture Media
If it's not contamination, particulates in culture often have an inorganic explanation. Understand balanced buffer and media constituents, and learn more about keeping media constituents in suspension.
Related Technical Articles
- Know when to use antibiotics to prevent bacterial or fungal, mycoplasma, or viral contamination in cell culture and find suitable antibiotics or other biological agents.
- Antibiotic kill curve is a dose response experiment in which mammalian cells are subjected to increasing amounts of selection antibiotic
- Cell culture antibiotic selection guide, including antibiotics for mammalian cell culture, plant cell culture, and antibiotic selection agents for cell culture.
- What is endotoxin? Frequently asked questions about bacterial endotoxin contamination of in vitro cell cultures. Details about how to endotoxin test using the LAL assay, common sources of laboratory endotoxin contamination and tips on how to avoid endotoxin contamination when culturing cell lines.
- See All (21)
Related Protocols
- This technical article covers the protocol for how to properly harvest cells from Corning® CellSTACK® culture chambers.
- Cell culture protocol for proper thawing of cryopreserved cell lines.
- Cell culture protocol for passaging and splitting adherent cell lines using trypsin EDTA. Free ECACC handbook download.
- Cell culture protocol for freezing cell lines at high cell viabilities using cryopreservation reagents such as DMSO. Cryopreservation is a method whereby cells are frozen, maintaining their viability, until they are defrosted months or years later. Free ECACC handbook download.
- Protocol for the seeding and cultivation of primary cells received in cryopreserved or proliferating formats. Information on primary cell culture, cell handling and passaging.
- See All (2)
To continue reading please sign in or create an account.
Don't Have An Account?