Tips for Optimizing Immunofluorescence Protocols
to Get the Best Image
Immunostaining with Fluorescent Antibodies
Uses for immunofluorescence (IF)—where an antibody is conjugated to a molecule that fluoresces when excited by lasers— include protein localization, confirmation of post-translational modification or activation, and proximity to/complexing with other proteins. Immunocytochemistry (ICC) is a well-established technique that uses antibodies to bind to targets in cell samples, and Coons et al first described immunodetection using a fluorescent reporter molecule in 1942. In addition to providing information about subcellular targets, combining immunofluorescence with immunocytochemistry produces some of the most compelling visual data in the life sciences. In this guide, antibody scientists share what we’ve learned about getting the best possible image from your IF-ICC experiments.
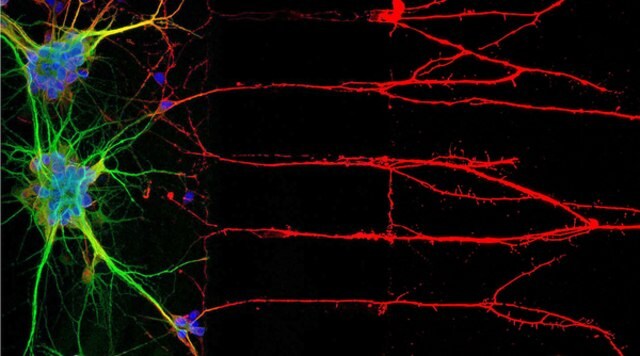

Figure 1: Immunocytochemistry (ICC) locates proteins associated with neuronal nuclei, soma, and axons (left). Right, immunofluorescent staining of human cell line U-251 MG.
- Sample Preparation
- Fixation
- Antigen Retrieval
- Permeabilization
- Antibody and Fluorophore Selection
- Antibody Selection: Staining Technique
- Antibody Selection: On-Target, Specific Reactivity
- Experimental Controls for IF-ICC
Fluorescent Immunodetection for ICC: Step by Step
IF-ICC follows some basic protocol steps, shown here for indirect staining:

Figure 2.Fluorescent immunodetection
Optimizing some steps of an immunofluorescence protocol may be needed in order to obtain the best results based on factors that include subcellular location of the target and antigen characteristics.
Sample Preparation:
Regardless of cell growth format, successful IF-ICC imaging starts with harvesting healthy samples at the appropriate culture density.
Suspension Cell:
Cell types may be washed to remove media and resuspended, then stained in small volume tubes before mounting onto slides for microscopic examination. Suspensions should first be examined microscopically for morphology and should not exceed the recommended confluency/titer for the cell line in order to be deemed suitable for immunocytochemistry. Healthy, subconfluent cultures will not be excessively turbid nor demonstrate yellowing indicating media acidification. Cells should appear bright and rounded by microscopic examination. Percent viability may be determined by hemocytometer and trypan blue exclusion. Following washing, cells should be resuspended at 1 -2 x 106 cells/mL, whether for ICC staining in solution, or smear application for subsequent staining on slides specially treated to enhanced adhesion of cells.
When adherent cells are cultured (and perhaps treated) to examine the effect on target expression by IF-ICC, cells may be seeded onto chamber slides, which are slides optimized for optical imaging with single- or multiple-chamber superstructures affixed that allow the addition of media for culture. Chamber slides permit transition directly from cell culture to probing and imaging. Adherent cell types may also be seeded onto microscope slide coverslips immersed in media-filled wells, which are subsequently stained and mounted onto slides for imaging.
Regardless of culture surface and vessel approach, cell growth on coverslips or in slide chambers should be carefully monitored, so that cells can be removed from culture and fixed for immunocytochemistry before they are overgrown. Cells are often imaged when confluency (the amount of surface area covered by cells) is in the 50-80% range. If cell viability must be definitively determined prior to immunostaining, some researchers have used dyes such as ethidium homodimer, a nuclear marker that cannot cross the intact cell membranes of live cells—so costaining with DAPI, which fluoresces in the nuclei of both viable and nonviable cells, can help to quantify percent viability as the number of ethidium-stained nuclei/number of dual-stained nuclei. Alternatively, commercial live/dead cell kits are available that often employ calcein and propidium iodide to distinguish live from dead cells, respectively.
Fixation:
Fixation and permeabilization may be achieved in a single step with organic fixatives like alcohol and acetone. Organic solvents should, however, not be used when lipid integrity must be maintained, as when the target antigen is associated with the membrane. Although formaldehyde (often sold and used in its polymeric form paraformaldehyde) is a common choice that halts decomposition and fixes proteins in place, crosslinking aldehydes can hinder antibody access to some antigens, and should therefore be used at the lowest concentration that effectively preserves structure after 10-20 minutes incubation, typically between 1-4% for (para)formaldehyde. NOTE: Because it is a source of autofluorescence, it’s best to avoid glutaraldehyde in IF fixation protocols.
Antigen Retrieval:
Some target epitopes may be masked by being complexed in a crowded cellular microenvironment, or by fixation techniques. Antigen retrieval techniques have been demonstrated to enhance access of antibodies to certain antigen targets, but should be used with extreme caution and pretested when working with cells on slides, as the heat or chemical conditions to which they expose cells are typically too harsh for ICC samples.
Permeabilization:
Triton-X or Tween-20 detergents are commonly used to effectively permeabilize fixed cells, but these detergents indiscriminately dissolve lipids and can be more destructive than saponin, a plant-based glycoside that perforates the membrane by selectively dissolving cholesterols, leaving organelle membranes essentially intact. If the target of interest is located within membrane bound structures inside the cell, more robust detergents such as Triton-X, Tween-20, the Brij detergents, or NP-40 are more likely to provide antibody access. Saponin is a gentler permeabilizer that preserves the integrity of surface antigens, but may not be suitable for permeabilizing intracellular membranes to provide antibody access to targets in the nucleus or other organelles. Because saponin’s effects may be reversible and therefore lost to washes, its use should be maintained throughout the staining protocol to ensure antibody access.
Blocking:
A solution of up to 5% normal serum from the species in which the secondary antibody is raised will effectively block its nonspecific binding. When serum is not available, an approach that adapts to any secondary is to use a more general protein blocker like bovine serum albumin (BSA) at a similar dilution in PBS. Some labs use a combination of serum and BSA to achieve both antibody host-specific and general blocking. BSA is often supplied lyophilized and reconstituted to liquid, so it should be filtered and/or regularly examined for particulates that may deposit on samples, creating staining artefact. Blocking may be maintained throughout the immunofluorescence protocol by diluting antibodies in the blocking buffer.
Antibody and Fluorophore Selection
Fluorophore Selection
When planning any fluorescence experiment examining cells by ICC, the first consideration should always be the optical configuration of the microscope to be used for analysis. IF-ICC protocols may be used to mark a single target, but many fluorescent microscope configurations can accommodate multiplexing, or the simultaneous staining of several targets, along with the nucleus. A sample experimental palette for staining three targets plus the nucleus is shown below:
Fluorescent microscopes will have unique configurations for excitation and emission. Microscopes are factory equipped with high intensity light sources such as arc lamps, or lasers that emit light at a specific wavelength capable of exciting compatible fluorophores. Filter sets capture a range of wavelengths of the light emitted by excited fluorophores, which may be broad to maximize the emission captured, or narrow to optimize specificity and reduce emission channel overlap. The table above is provided as an example based on common fluorescent microscope configurations. Please consult your instrument laser and filter configurations to help you choose the best fluorophore-conjugated antibodies for your immunofluorescence ICC or IHC protocol. Microscopy core scientists are often a helpful resource for IF experiment design.
Additional Considerations when Selecting Fluorophores
Photostability: Photobleaching, or irreversible light-induced damage to fluorophores affecting their ability to fluoresce, can result from overexposure of fluorescent molecules to ambient light or excitation during microscopy. Many ubiquitous fluorophores such as FITC and TRITC are widely available because they are non-proprietary, and due to their market longevity—but may be much more sensitive to photobleaching than later-generation fluorophores that use other chemistries.
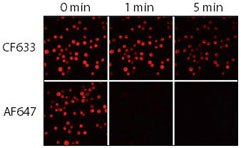
Figure 3.Relative photostability of CF633 and Alexa Fluor 647 (AF647) goat anti-mouse conjugates. Jurkat cells were fixed, permeabilized and stained with rabbit anti-CD3 followed by CF633 or Alexa Fluor® 647 goat anti-rabbit IgG conjugates. Samples were imaged using a mercury arc lamp microscope equipped with a Cy5 filter set and CCD camera. Sequential images were captured at 0, 1, and 5 minutes.
When producing diluted antibody working solutions containing fluorescent probe, consider diluting this working solution in a light-shielding container such as an amber vial. Working solutions are often exposed to light for longer periods of time than stored stock solutions or commercial antibody preps, so this extra measure that reduces light exposure may provide additional protection in use scenarios.
Fluorescence Protection Program: Extend the Lifetime of Your Fluorescent Antibodies and Reagents
Fluorescent reagents may require special storage and handling, to maintain the photostability of the fluorescent entity as well as the functional integrity of the antibody itself. Ensure that the reagent arrives under any temperature conditions specified in the supplier’s shipping documents, and transfer to laboratory storage recommended by the manufacturer so that light and temperature conditions are not compromised. Aliquoting antibody stock on arrival into smaller volumes (with replicated labeling) protects reagent integrity by limiting the number of times the stock solution is exposed to freeze-thaw, ice bucket variations or other temperature changes, or ambient light.
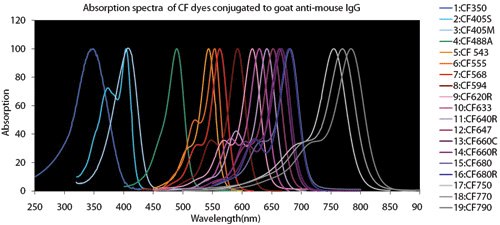
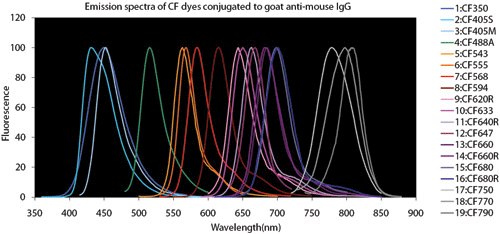
Figure 4.Fluorescent Dye Comparison Charts
Spectral overlap: When performing multiplex experiments to detect multiple targets in the same sample, refer to fluorophore spectral characteristics, laser wavelengths, and microscope filter ranges. Microscope emission filter configurations should be selected to reduce spectral overlap, but choosing fluorophores that do not have long emission ‘tails’ that may bleed into an adjacent filter channel is good practice.

Figure 5.Relative fluorescence of CF543 and Alexa Fluor 546 (AF546) goat anti-mouse conjugates as a function of the number of dye molecules per protein (degree of labeling).
Antibody Selection: Staining Technique
Antibody Selection
Direct staining: Some researchers may appreciate the convenience of a ‘direct staining’ approach, in which primary antibody is directly conjugated to a fluorophore, as binding and fluorescent detection are achieved in a single step, and the overall time needed to complete the IF-ICC protocol is reduced. Direct conjugates also facilitate multiplex staining of multiple targets in the same sample by eliminating the need to choose primary antibodies from different hosts to avoid cross-reactivity (more about multiplex IF-ICC, below).
If a primary antibody conjugate is used, this step should be conducted in the dark to protect the fluorophore from photobleaching. Following incubation with the primary conjugate, the protocol can proceed immediately to washing and mounting. A cocktail of antibodies may be used to stain multiple targets in the sample in a single step.
Indirect staining method: In ‘indirect’ immunodetection, an unlabeled primary antibody specific for the target is then followed by a fluorophore-conjugated secondary antibody that detects the host species of the primary antibody. This technique confers the distinct advantage of signal amplification, as there is the potential for multiple fluorophore-bearing secondary antibodies to bind the primary antibody, increasing the number of fluorescence-emitting molecules at the site of antigen.
Multiplex targeting of multiple antigens simultaneously in the same sample requires additional considerations with the indirect detection method, as explained below.
Antibody selection: on-target, specific reactivity
Primary antibody:
During indirect detection, antibodies will be applied in a cocktail for multiplex experiments, both at the primary and secondary incubation steps. It is therefore essential to choose primary antibodies for each target that were raised in a different host. This ensures that each conjugated antibody in the secondary cocktail binds to a unique primary antibody in the sample. Failure to select unique hosts for each target antibody will result in uninterpretable staining results. Below is a sample experimental setup for multiplex indirect detection:
In this example, if the researcher instead chose a rabbit anti-B primary antibody, both anti-A and anti-B would be targeted by the anti-rabbit secondary conjugate, and both targets A and B would be marked by green fluorescent signal.
Primary antibody-tissue cross-reactivity: When choosing antibodies for indirect staining, it is advisable to deselect any primary antibodies raised in the same host from which the sample is derived---for example, don’t use a mouse primary on mouse tissue. Following with an anti-mouse secondary would likely result in widespread binding of the secondary antibody directly to the tissue, causing significant background staining. Although kits are sold that are intended to block host-on-self reactivity, the most risk-free course of action is to avoid host-on-self when choosing primary antibodies for indirect protocols. NOTE: host-on-self cross-reactivities are generally not an issue for direct staining approaches.
Secondary Antibody:
As explained above (Antibody selection: staining technique, the indirect method, above) ‘indirect’ IF-ICC makes signal amplification possible, as multiple secondary antibodies may bind primary antibodies bound to the target. But indirect IF-ICC also allows the lab to build a modular library of secondary antibodies that will be compatible with a growing collection of primary antibodies, so that any target can be assigned to any available microscope filter channel.
Washing:
Washing after each application of antibody or other fluorescent probe eliminates antibodies with lower binding affinity present in the sample and thus reduces non-specific signal, or cross-reactivity. The importance of the wash step cannot be overstated. Washing for a few minutes in PBS with at least two buffer exchanges will help to eliminate unbound and loosely-bound antibody from the sample.
Longer washes may not confer noticeable background-reduction benefit, but are generally not harmful so long as extended time in buffer does not detach the sample from the slide or coverslip. Very short washes with less repeats may fail to effectively remove antibody that is not specifically bound to target, which can lead to poor signal:background ratio.
Nuclear Counterstain and Mounting:
Many researchers performing IF-ICC may wish to use a fluorescent reagent that marks the cell nucleus, such as DAPI or Hoechst. Because both of these intercalate to become fluorescent within seconds of accessing the DNA, mounting medium that contains DAPI can be used to achieve nuclear stain and mounting in a single step. This saves time by eliminating the need for separate DAPI/Hoechst staining and washing steps, and reduces variability in the concentration and incubation time for the nuclear counterstain.
All mounting media help affix the coverslip to the slide, and preserve the sample for microscopy by preventing dehydration. Many mounting media are also formulated to optimize the refractive index for microscopy, and may contain agents that protect the fluorophores bound to the stained sample from photobleaching.
Experimental controls for IF-ICC
As with other experiments, including positive and negative controls will enhance confidence in the sensitivity and specificity of fluorescent ICC results.
Controls for IF-ICC experiments may include:
Omit the primary:
A simple control that can easily be included in any ICC experiment is one where the primary antibody is omitted from the primary staining step in an indirect immunodetection protocol. This demonstrates whether any signal observed may be due to nonspecific binding of the fluorescent secondary antibody directly to the sample.
Isotype Control:
For direct staining with conjugated primary antibodies, a sample where an isotype control conjugated to the same fluorophore is substituted for the primary conjugate helps to ensure that any signal observed is due to specificity of the primary antibody for the antigen. Isotype controls are often available from the supplier of the antibody conjugate, and are of the same class and immunoglobulin type as the antibody against the antigen, but have no specificity for any known epitope. Isotypes serve as a control for the primary antibody, as their inclusion demonstrates that any signal observed is not due to the mere ‘stickiness’ of the primary antibody protein itself.
Cells Negative for the Target Antigen:
A cell type may be known not to express the antigen of interest and can serve as a negative control sample. Alternatively, cells with the target of interest genetically modified so that the protein of interest is not expressed (knock-out cells) are a robust control for target specificity, if they are available. Genetic modification techniques such as CRISPR/Cas nuclease may be used to knock out expression of the target protein to create a negative control cell line.
Cells Positive for the Target Antigen:
If cells are available that have been previously shown to express the antigen of interest, whether endogenously or by modification to ‘knock in’ or overexpress the gene, these may serve as a positive control to confirm that the staining protocol produces signal when the target is present in the sample.
Insider Advice for Getting the Best Signal—and Conquering Background—with IF-ICC
- Whenever possible, choose antibodies where data is available demonstrating results in ICC applications
- Match fluorescent antibody spectral characteristics to microscope lasers and filters
- Fix cell samples that are healthy, free of contamination and precipitates, and sub-confluent
- Optimal fixation and permeabilization method depends on the target and its subcellular location
- Dilute antibodies in blocking buffer to maintain blocking throughout staining protocol
- Indirect detection with a fluorescent secondary antibody permits signal amplification
- When choosing from amongst antibody conjugates with similar spectral characteristics, choose the more stable fluorophore to avoid photobleaching during long or repeated microscope exposures—and avoid spectral overlap
- Always include appropriate staining controls. Use positive and negative biological controls when available.
References
Per continuare a leggere, autenticati o crea un account.
Non hai un Account?