Preparing CHO Cells for Higher Productivity by Optimizing a Perfused Seed Train
Michael Cunningham, Jana Mahadevan, Mona Bausch
The upstream seed train expansion process sets the stage for biotherapeutic production and offers opportunities to improve manufacturing efficiency, increase productivity and reduce costs. Existing upstream equipment and/or facility infrastructure can, however, present an impediment to intensification efforts and consequently limit gains in efficiency. Productivity limitations with fed-batch production bioreactors can occur where maximum achievable cell densities are limited. One strategy for increasing output from an existing fed-batch production bioreactor is to use perfusion technology in the N-1 bioreactor to increase the biomass generated in the seed train. This additional biomass enables inoculation of the fed-batch bioreactor at much higher cell densities than those commonly used in conventional fed-batch processes.
This application note describes the benefits and performance of Cellvento® 4CHO-X Expansion Medium designed for multiple cell expansion steps from vial thaw to N-1 stages to generate higher biomass for inoculating fed-batch production bioreactors. Data showing the doubling time, viability and viable cell densities of four Chinese hamster ovary (CHO) cell lines grown in the expansion medium are presented, which demonstrate the feasibility of this application with an off-the-shelf, ready-to-use product. This cell culture medium also eliminates the need to perform cell adaptation or compatibility confirmations when switching from this medium to one of our platform media in the production stage.
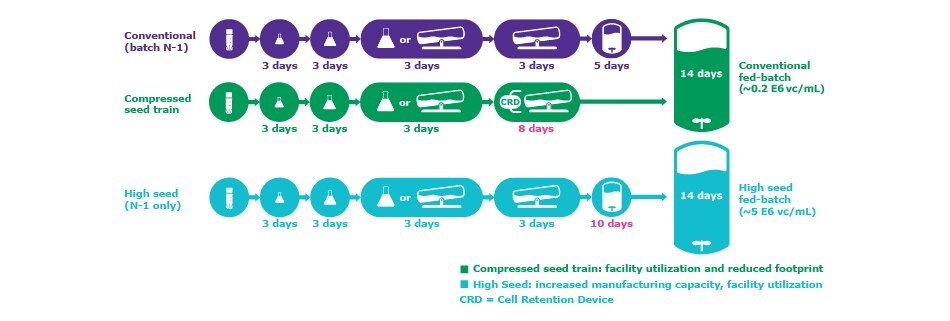
Figure 1.Comparison of a standard manufacturing process to processes that use N-1 perfusion in the seed train.
Benefits of N-1 Perfusion and Purpose-Built N-1 Expansion Medium
The seed train is essential in scaling to a sufficient cell density for inoculating a production bioreactor. Historically, the seed train has relied heavily upon batch or fed-batch processes in which cells are accumulated over the course of several, increasingly larger volumes, and then used as an inoculum for the production bioreactor. The cell densities generated by these approaches have been constrained due to the small volumes of the culture vessels and the cell growth limitations resulting from conventional feeding strategies offering limited nutrition. Use of perfusion at the N-1 step and Cellvento® 4CHO-X Expansion Medium, allows generation of a higher biomass accumulation; this allows the fed-batch production bioreactor to be inoculated at 5–15 fold the cell densities that can be achieved in a conventional N-1 batch process (Figure 1). Here it is important that cells remain in exponential growth phase to be suitable for the inoculation of the final N-stage bioreactor.
Cellvento® 4CHO-X Expansion Medium was developed for seed train expansion and N-1 perfusion of CHO cell lines and supports high growth and viability in the N-1 bioreactor, thus increasing cell biomass available for inoculation of the production bioreactor (Figure 2). The expansion medium is formulated with an optimal nutrient concentration to achieve low cell specific perfusion rates (CSPRs) at high cell densities. The medium is chemically defined, of non-animal origin, and contains no hydrolysates or components of unknown or variable composition. The medium has been specifically formulated to control the metabolic profile of the cells in a perfusion mode of operation, both to minimize inhibiting byproducts and also to ensure adequate nutrient levels to support high cell densities.
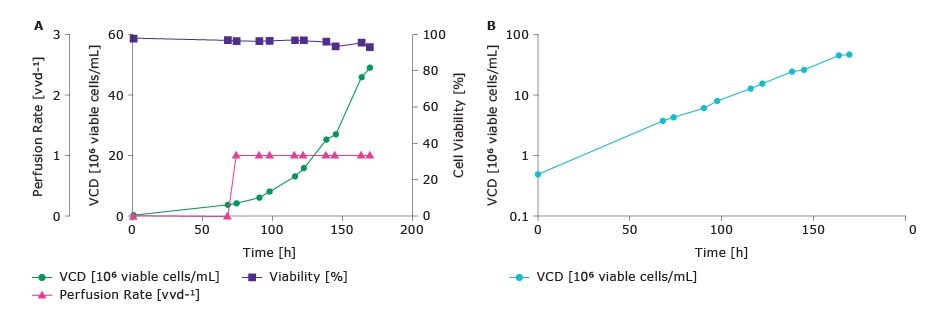
Figure 2.(A) A bioreactor was inoculated with a CHOZN® GS cell line at 0.5 x 106 viable cell/mL and grown in batch mode in Cellvento® 4CHO-X Expansion Medium for approximately 3 days. After this period, perfusion was initiated using a perfusion rate of vvd-1 with Cellvento® 4CHO-X Expansion Medium supplemented with glucose. (B) Logarithmic plot of VCD to demonstrate cells in N-1 perfusion bioreactor run were maintained in exponential growth phase.
Performance of Cellvento® 4CHO-X Expansion Medium
For this study, commonly used CHO cell subtypes (CHO-GS, CHO-K1-GS and CHO DG44) were used to demonstrate the broad applicability of Cellvento® 4CHO-X Expansion Medium. Cell lines were initially cultured in Cellvento® 4CHO-X Expansion Medium and then seeded at 300,000 cells/mL into TPP spin tubes with a 30 mL working volume. The spin tubes were placed in a 37 °C shaker incubator supplied with 5% CO2, 80% humidity, 25 mm throw at 320 rpm. Cells were then passaged after 3 or 4 days.
Figure 3 shows the doubling time (A), viability on day 4 (B) and viable cell density (C) on day 4 of two CHOZN® GS-/- clones (CHOZN-GS 1 and CHOZN-GS 2) and two other commonly used production cell lines (CHO-K1-GS and CHO DG44) grown in Cellvento® 4CHO-X Expansion Medium. All four cell lines showed consistent doubling times as well as viabilities and viable cell densities on day 4 of the culture. Table 1 shows the same cell lines and the doubling times on day 3 and 4 – with no significant differences observed.

Figure 3.(A) Day 4 Doubling time, (B) Day 4 Viability and (C) Day 4 Viable Cell Density of four CHO cell subtypes grown in Cellvento® 4CHO-X Expansion Medium.

Table 1. Cell culture profile of various CHO subtypes in Cellvento® 4CHO-X Expansion Medium.
One important consideration, when using N-1 perfusion, is that the medium in the N-1 perfusion bioreactor may be different from the medium used for the N-stage. In this case, compatible media that do not require adaptation are beneficial. The Cellvento® 4CHO-X Expansion Medium was designed to be used throughout the seed train and is compatible with our EX-CELL® and Cellvento® fed-batch and perfusion production media offerings. This convenient feature eliminates the need to perform cell adaptation, or even check for compatibility. Cellvento® 4CHO-X Expansion Medium enables elimination of a time- and resource-intensive adaptation step when moving to the production steps; the workflow can progress from vial thaw to production without the need for media adaptation if the cell bank is done in Cellvento® 4CHO-X Expansion Medium.
The compatibility of Cellvento® 4CHO-X Expansion Medium with fed-batch platforms Cellvento® 4CHO/4Feed and EX-CELL® Advanced fed-batch is shown in Figure 4. Given that different CHO cell subtypes yield different growth characteristics profiles, we evaluated many of them for a more holistic/comprehensive analysis of the utility of Cellvento® 4CHO-X Expansion Medium. Presented here are two CHO cell lines that were expanded either in the Cellvento® 4CHO Medium and EX-CELL® CD Fusion medium for these platforms or in Cellvento® 4CHO-X Expansion Medium.
The conditions expanded in the Cellvento® 4CHO-X Expansion Medium are shown in Figure 4 below with a dashed line; conditions expanded in the conventional basal medium (baseline control) are shown as solid lines. With the Cellvento® 4CHO platform, the expansion medium had a significant titer-increasing effect; with EX-CELL® Advanced fed-batch media platform, with these two clones, the Cellvento® 4CHO-X Expansion Medium performed comparably. These results demonstrate the Cellvento® 4CHO-X Expansion Medium can also be used when using either Cellvento® 4CHO or EX-CELL® Advanced fedbatch media in the final production step. Volumetric titer levels varied amongst the several cell line and media conditions evaluated, reflecting that some cell lines were more sensitive to the media formulations.
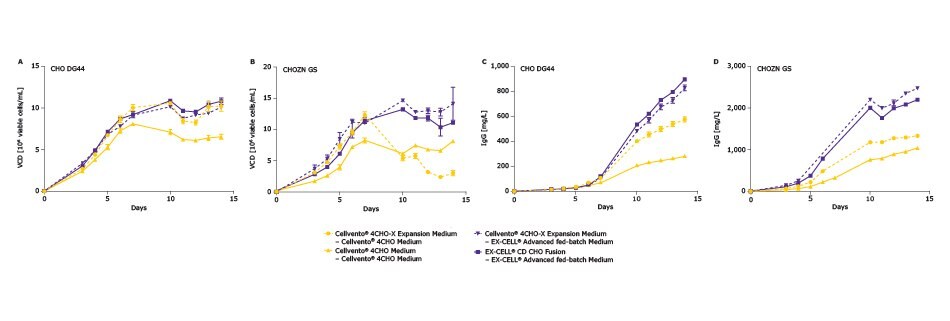
Figure 4.Compatibility of expansion medium with Cellvento® 4CHO and EX-CELL® Advanced CHO fed-batch Media. The first medium listed indicates expansion medium used and second medium listed indicates fed-batch medium used. (A) Viable cell density for CHO DG44 subtype. (B) Viable cell density for CHOZN® GS subtype. (C) IgG titers for CHO DG 44 subtype. (D) IgG titers for CHOZN® GS subtype.
TPP results shown in Figure 4 were then confirmed in a 1 L bioreactor (Figure 5). The Cellvento® 4CHO platform was tested for compatibility with Cellvento® 4CHO-X Expansion Medium for each condition. The purple condition shows cell expansion in the Cellvento® 4CHO medium; the yellow condition shows cell expansion in the Cellvento® 4CHO-X Expansion Medium. Following these separate seed train expansions, the cell cultures were then used to inoculate the fed-batch production bioreactors using the Cellvento® 4CHO fed-batch platform. While both conditions yielded similar cell growth and viability profiles, the seed train expansion employing Cellvento® 4CHO-X Expansion Medium showed significantly higher titer compared to Cellvento® 4CHO medium.

Figure 5.Bioreactor confirmation of compatibility with Cellvento® 4CHO fed-batch platform showing a 36% increase in titer.
With this study, compatibility with our EX-CELL® and Cellvento® fed-batch and perfusion production media offerings could be shown. To evaluate the benefits of the N-1 application itself, a N-1 bioreactor was run with Cellvento® 4CHO-X Expansion Medium. This bioreactor was then used to inoculate a production bioreactor with EX-CELL® Advanced medium and a 50:50 ratio of EX-CELL® Advanced Feed and Cellvento® 4Feed utilizing one of two seeding density options. Mobius® bioreactors (3 L with 1.5 L working volume) were inoculated at either a low (0.5 × 106 cells/mL) or high (5.0 × 106 cells/mL) seeding density, and subsequently operated in fed-batch mode utilizing similar feeding schedules. In addition, a control fed-batch production (N) condition, where cells were obtained from a conventional, non-perfusion seed train expansion utilizing Cellvento® 4CHO-X media in shaker flasks and used to seed a low density (0.5 × 106 cells/mL) reactor, was operated in parallel. Each bioreactor study condition was evaluated in duplicate. Results shown in Figure 6 demonstrate:
- Cell performance in Cellvento® 4CHO-X Expansion Medium was not negatively impacted by utilization of perfusion at the N-1 stage (demonstrated by similar cell viabilities and specific productivities in all evaluated conditions).
- High cell density inoculation of the production (N) bioreactor yielded significantly higher levels of both total biomass and accumulated volumetric titer over the study time course.

Figure 6.Comparability of Cellvento® 4CHO-X performance in a production (N) bioreactor utilizing cell inoculation at low (0.5 x 106 cell/ml) and high (5 x 106 cell/ml) cell densities. Cell performance was compared by viable cell density (A), cell viability (B), accumulated volumetric titer (C) and specific productivity (D).
Seeding the fed-batch production bioreactor at a higher cell density generated by the combination of N-1 perfusion and Cellvento® 4CHO-X Expansion Medium delivers important benefits:
- Significantly reduces the production bioreactor operating time (days required to reach its maximum cell density).
- Decrease facility footprint by eliminating one bioreactor in your seed train, or alternatively, allows for inoculation of multiple traditional fed-batch production bioreactors from a single high cell density N-1 perfusion bioreactor, which can help optimize utilization and productivity of existing manufacturing infrastructure.
Conclusion
Upstream process intensification has the potential to increase productivity, accelerate protein production and improve efficiency of existing facility footprints. Implementation of perfusion-based processes, especially in the seed train, can reduce the costs of manufacturing and increase product throughput, all while maintaining the production bioreactor in a more simple-to-operate fed-batch mode. N-1 perfusion using Cellvento® 4CHO-X Expansion Medium supports inoculation of the production bioreactor at a much higher cell density, which can result in increased product titers. Results for the CHO cell lines evaluated in this study provide evidence of both process robustness as well as operational flexibility with several CHO cell subtypes with the Cellvento® 4CHO-X Expansion Medium.
Per continuare a leggere, autenticati o crea un account.
Non hai un Account?