Fast and high-resolution Analysis of Intact Therapeutic Monoclonal Antibody Trastuzumab using BIOshell™ A400 Protein C4 Column
Sundaram Palaniswamy, Segment Lead, Pharma QC, Aditya Pratihast, Application Scientist
Merck
Abstract
Although the majority of small molecules analysed by reversed phase have a mass below 1500 Da, there is a growing need to improve the performance of HPLC columns for the separation of therapeutic proteins and protein drug conjugates. This application note demonstrates a fast and reproducible reversed phase method with high-resolution for the analysis of intact therapeutic monoclonal antibody, trastuzumab. Separation and quantification were achieved using a BIOshell™ A400 Protein C4 column in less than 5 minutes, and more importantly, the optimized method was able to monitor degradation compounds created by heat stress studies.
Introduction
Over the past few years, monoclonal antibodies (mAbs) have become the best-selling drugs in the pharmaceutical market, and in 2018, eight of the top 10 best-selling drugs worldwide were biologics. The global therapeutic monoclonal antibody market was valued at approximate $115 billion in 2018 growing up to $300 billion by 2025. And although as of December 2019, 79 therapeutic mAbs have been approved by the US FDA for sales worldwide, there is a significant potential for the number to increase.1 HPLC is a well-established method for the analysis of intact mAbs by Size Exclusion and Ion Exchange chromatography. However, technological advancements in the field of Reversed Phase (RP) have made them promising tools for the analysis on intact proteins.2 Intact mAbs are yet analyzed with limited success using wide pore, fully porous particles due to their large molecular size leading to slow mass transfer and long analysis times. Superficially porous particles have overcome these challenges, but have lower loadability and still a rather limited offering of different stationary phases. Moreover, highly resolving core-shell columns easily separate intact mAbs quickly and with high efficiency.
Here, we have demonstrated the suitability of the BIOshell™ A400 Protein C4 column for a fast and high-resolution separation of intact trastuzumab using RP- HPLC. The retention time and area precision of the method were excellent, demonstrating the suitability of the column. Further, we also showcase quantification and robustness that is highly suitable for biopharmaceutical QC applications.
Experimental
Equipment and Sample
The study was performed on a Shimadzu LC-2010CHT HPLC System. The therapeutic trastuzumab was purchased from a local pharmacy.
Methods
Chromatographic parameters for intact trastuzumab using a BIOshell™ A400 Protein C4 column are shown in Table 1.
Linearity, Limit of Quantitation (LOQ) and Limit of Detection (LOD)
The calibration curve was constructed with nine standard concentrations of trastuzumab from 1 to 25 µg/mL. The mAb concentration that provided a signal-to-noise ratio (S/N) > 3 was considered LOD and S/N > 10 was considered as LOQ.
Forced Degradation Studies
We compared the chromatographic profiles of native and heat-stressed trastuzumab for monitoring degraded products. For the forced degradation studies, 1 mg/mL of trastuzumab was exposed to 10 ppm hydrogen peroxide (H2O2) followed by heating at 80 °C for 60 min. An aliquot of 10 µL was used for RP HPLC analysis.
Results and Discussion
Intact Trastuzumab Analysis
For the HPLC analysis, a BIOshell™ A400 Protein C4, 3.4 μm HPLC Column with core-shell particles and 400 Å pore size delivered reproducible, fast, and high-resolution separation of intact trastuzumab, making it suitable for biopharma development and QC applications. Figure 1 demonstrates excellent peak shape and overlays of six replicates in less than 5 minutes under the chromatographic conditions.
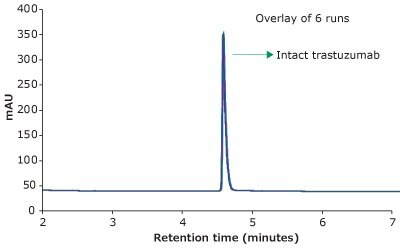
Figure 1.RP-HPLC analysis of trastuzumab on a BIOshell™ A400 Protein C4, 100 x 2.1 mm, 3.4 µm HPLC column.
Precision of Retention Time and Area
Table 2 shows the average Retention Time (RT) and Area RSDs from six replicates of trastuzumab injections. The Retention Time and Peak Area RSDs were less than 0.1% and 0.29 %, respectively, which demonstrates excellent reproducibility of the method and, thus, the precision of the method.
Limit of Detection and Limit of Quantitation
The LOD and LOQ were 0.125 µg/mL and 0.25 µg/ mL, respectively, for trastuzumab, indicating that the method was sensitive. Observed LOD and LOQ values of trastuzumab are reported in Table 3. Representative chromatograms on the same scale for 2 calibration runs & blank are shown overlayed in Figure 2.

Figure 2.Overlay of representative chromatograms on the same scale for 2 calibration runs & blank.
Linearity
Linearity curves for trastuzumab were constructed from 1 µg/mL up to 25 µg/mL in this study using area response and concentration of trastuzumab. The average peak areas are listed in Table 4. The linearity curve for trastuzumab is shown in Figure 3.
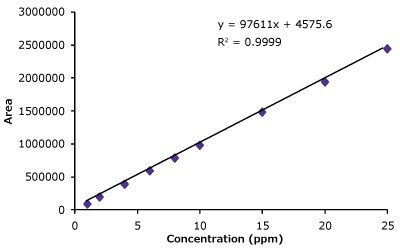
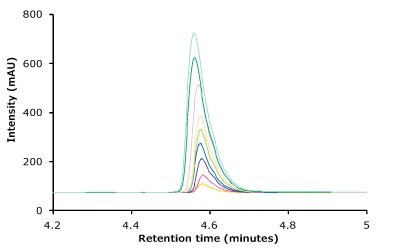
Figure 3.Linearity curve with nine standard concentrations of trastuzumab ranging from 1 to 25 µg/mL showing excellent coefficient values. Also shown are chromatogram overlays for the linearity ranges.
Trastuzumab Degradation Studies
We compared the intact and stressed trastuzumab using RP-HPLC to evaluate if this method is stability indicating. Any deviations in peak RT or Area as a result of stress were considered degradation products.
Figure 4 compares the RP-HPLC profile of unstressed and heat stressed trastuzumab. The profiles indicate that the BIOshell™ A400 Protein C4, HPLC column was able to distinguish between unstressed and stressed trastuzumab based on the peak shape and area.
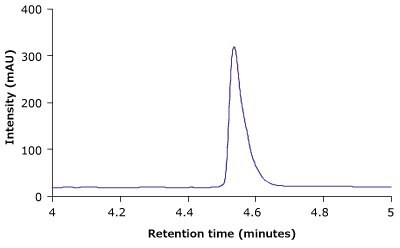
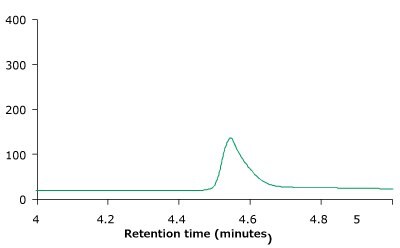
Figure 4.BIOshell™ A400 Protein C4, 100 x 2.1 mm, 3.4 µm RP-HPLC profiles of unstressed (top) and heat stressed trastuzumab sample (bottom).
Conclusion
Analysis of intact mAbs provides a first level of interrogation of size, post-translational modification and heterogeneity. RP-HPLC analysis of mAbs requires large pore sizes, a hydrophobic stationary phase, and appropriate chromatographic methods. In this application note a simple LC-UV method for the analysis of intact trastuzumab was showcased. Using a BIOshell™ A400 Protein C4 column, a high-resolution and rapid separation of intact trastuzumab was developed. Area and RT precision of the method were excellent and showed the reliability of method. The calibration curves with nine standard concentrations of trastuzumab had excellent coefficient of linearity values displaying that the method was quantitative and accurate. The LOD and LOQ for trastuzumab were found to be 0.125 µg/mL and 0.25 µg/mL, respectively, indicating the method was sensitive. In addition, heat stressed studies demonstrated that the BIOshell™ A400 Protein C4 column was able to monitor degraded mAbs and the method could be used for stability studies
References
Per continuare a leggere, autenticati o crea un account.
Non hai un Account?