N-Heterocyclic Carbene (NHC) Ligands
ChemFiles 2006, 6.8, 14.
Emerging Class of Privileged Ligands
Product Highlights
- Mediate an incredible breadth of transformations
- Provide robust stability to the metal center
- More active in many reactions than the related phosphine catalysts
- Readily modified to incorporate chirality, immobilization, and H2O solubility
NHC (N-heterocyclic carbenes) have evolved to become powerful, universal ligands for the rapid synthesis of novel organometallic complexes.1 In particular, NHC ligands have found practical use when bound to metals that are know to display high catalytic activity in related organophosphane metal coordination chemistry.2-4 Heterocyclic carbenes are emerging as a privileged class of ligands due to several attractive features including: 1) facile tuning of the electronics and/or sterics of a metal catalyst system; 2) ease of synthetic preparation from conveniently available starting materials; and 3) exhibit strong binding to the metal thus creating a highly productive catalyst. we are pleased to offer a diverse array of NHC ligands that have been successfully applied in reactions ranging from amination5 to C–C bond formation6 to hydrosilylation.7
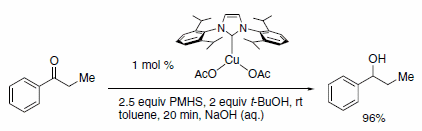
Figure 1.
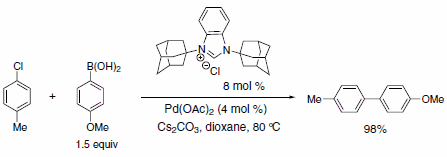
Figure 2.
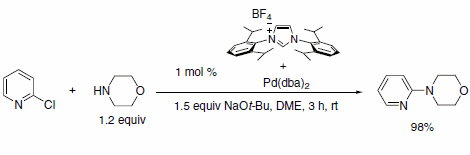
Figure 3.
References
Per continuare a leggere, autenticati o crea un account.
Non hai un Account?